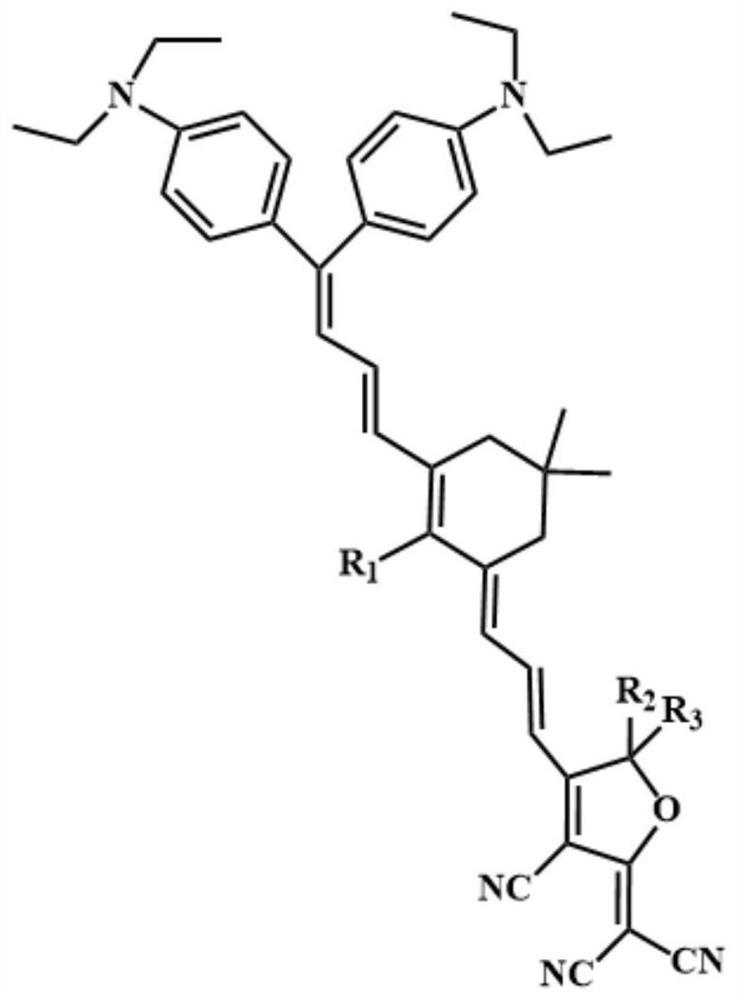
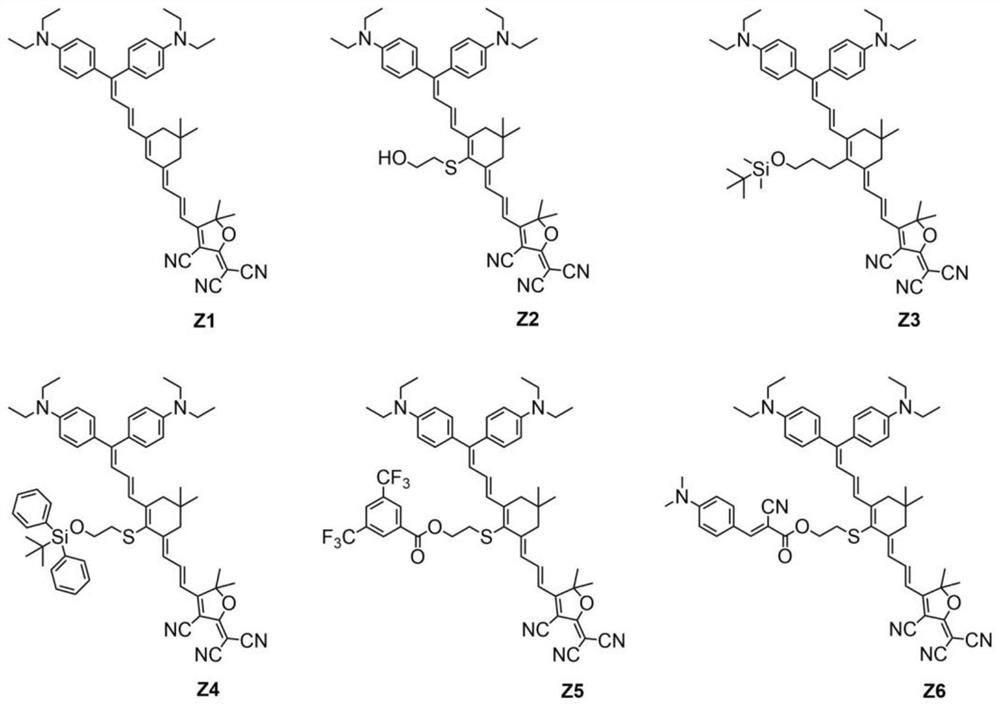
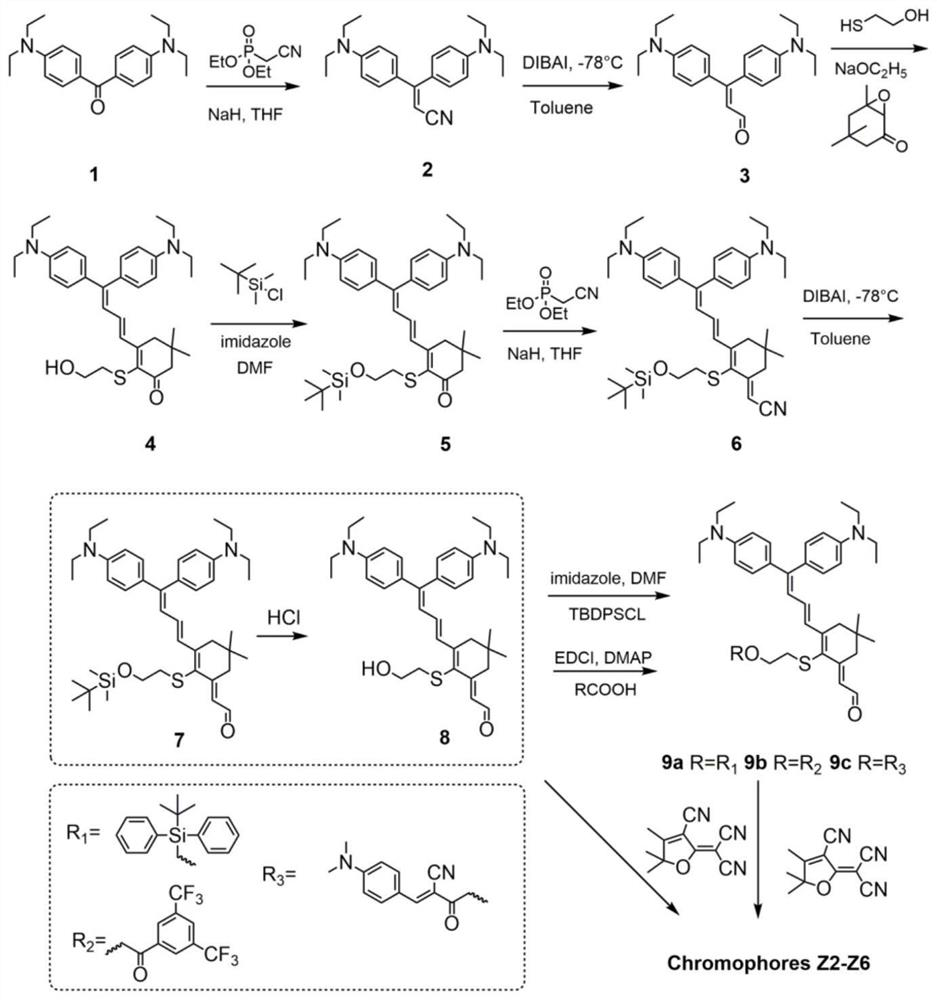
Double-donor organic optical nonlinear chromophore modified by thio-isophorone bridge as well as synthesis method and application of double-donor organic optical nonlinear chromophore
A technology of optical nonlinearity and synthesis method, which is applied in the field of dual-donor organic optical nonlinear chromophore and its synthesis, and can solve the problem that functional groups cannot be further modified.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] A dual-donor organic optical nonlinear chromophore modified by a thioisophorone bridge, the synthesis method comprising the following steps:
[0075] S1. Under N2 atmosphere, diethyl(cyanoethyl)-phosphonate (16.12 mL, 18.22 g, 102.7 mmol) was slowly added to a flask filled with sodium hydride (4.08 g, 102.7 mmol) and 40 mL of dry tetrahydrofuran. Compound (1) (15 g, 25.6 mmol) was then added to THF (90 mL) and refluxed overnight. After removing tetrahydrofuran in vacuo, it was directly purified by silica gel elution column chromatography with ethyl acetate / n-hexane (1:15-1:10) to obtain compound (2) in 70% yield (10.91 g, 17.9 mmol). MS(MALDI)(M+,C 23 H 29 N 3 ):calcd:347.29;found:347.23. 1 H-NMR (400MHz, acetone-d6): d=7.33–7.26 (m, 2H, Ar–H), 7.26–7.17 (m, 2H, Ar–H), 6.78–6.73 (m, 2H, Ar–H) ), 6.72–6.67 (m, 2H, Ar–H), 5.48 (s, 1H, CH), 3.46 (m, 8H, CH) 2 N),1.18(dt,J=10.4,7.0Hz,12H,4Me). 13 C-NMR (100MHz, acetone-d6): d=163.64, 150.23, 149.81, 132.25, 131.17, 1...
Embodiment 2
[0091] A dual-donor organic optical nonlinear chromophore modified by a thioisophorone bridge, the synthesis method comprising the following steps:
[0092] P1, diethyl (cyanomethyl)-phosphonate is slowly added to the tetrahydrofuran solution of sodium hydride, the compound (1) is added, and a reflux reaction is carried out under a protective atmosphere. After the reaction is completed, the solvent is evaporated and passed through Purification by silica gel chromatography, eluting with a mixed solvent of ethyl acetate and hexane, gave compound (2);
[0093] P2. Slowly add the hexane solution of diisobutylaluminum hydride to the toluene solution of the compound (2), react for a period of time at -78°C under a protective atmosphere, add water-containing wet silica gel, and continue at 0°C After the reaction is completed, the product is added to water, extracted with ethyl acetate, purified by silica gel chromatography after concentration, and eluted with a mixed solvent of ethyl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption wavelength | aaaaa | aaaaa |
| electro-optic coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com