Process and equipment for recycling nitrogen trifluoride electrolytic residue resources
A nitrogen trifluoride and resource recovery technology, applied in the direction of improving process efficiency, calcium/strontium/barium fluoride, ammonium halide, etc., can solve the problems of high energy consumption, waste of resources, complicated treatment methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
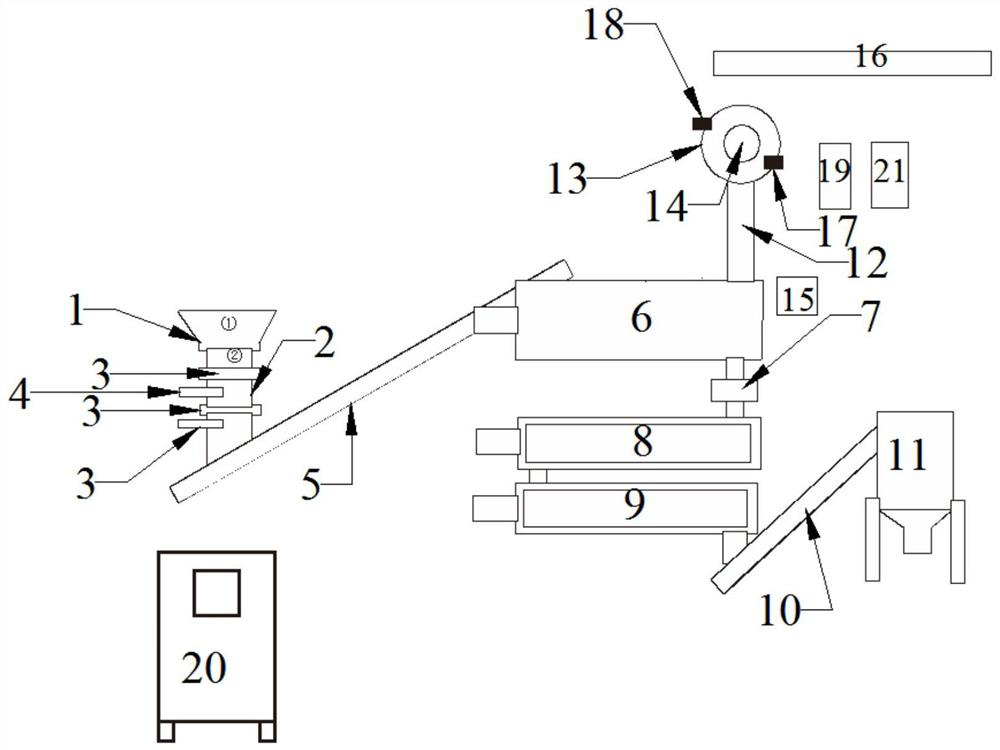
[0036] A device for recycling nitrogen trifluoride electrolysis residue resources, such as figure 1 As shown, the use method of the equipment is as the above-mentioned process of nitrogen trifluoride electrolysis residue resource recovery. The calcium oxide tank 16, the steam defluorination device 8, the ammonia water tank, the heat exchange device 21 and the cooling device 9; the above-mentioned devices are all controlled and operated by the control cabinet 20;
[0037] The outlet of the crushing device is connected with the inlet of the feeding device, the outlet of the feeding device is connected with the inlet of the high-temperature anaerobic pyrolysis device 6, and the gas outlet of the high-temperature anaerobic pyrolysis device 6 is cracked through the The gas pipe 12 is connected to the air inlet of the condensing device 14, the condensing liquid outlet 18 of the condensing device 14 is connected to the condensing liquid inlet 17 of the crystallization device 13 throu...
Embodiment 2
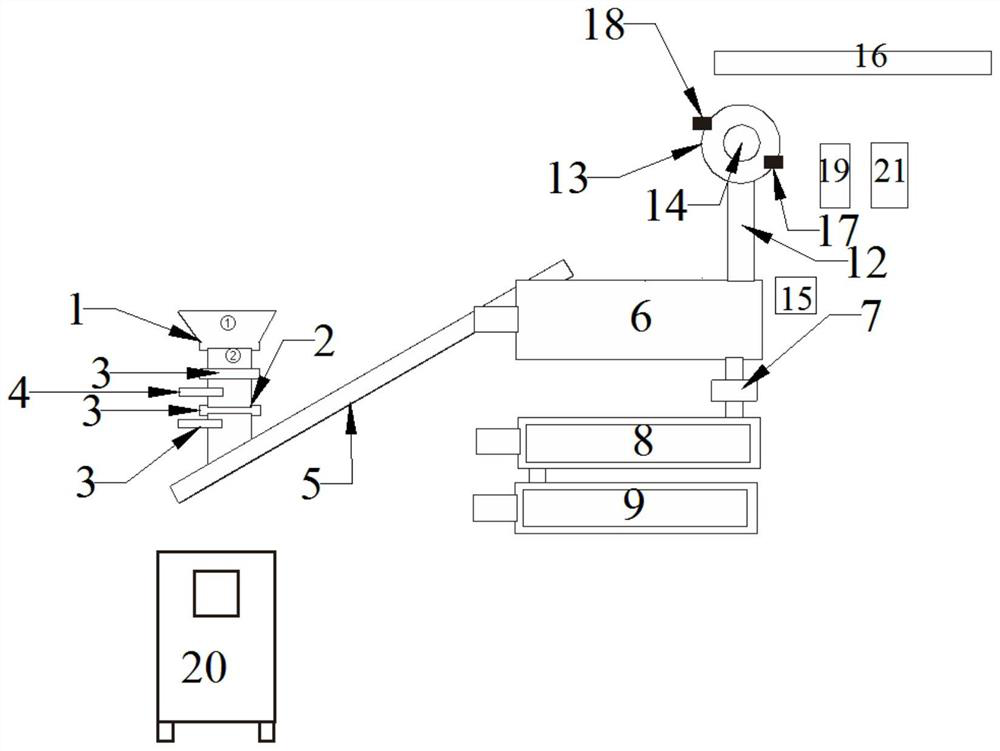
[0046] A device for recycling nitrogen trifluoride electrolysis residue resources, such as figure 2 As shown, the use method of the equipment is as the above-mentioned process of nitrogen trifluoride electrolysis residue resource recovery. The calcium oxide tank 16, the steam defluorination device 8, the ammonia water tank, the cooling device 9, the heat exchange device 21 and the discharging device; the above-mentioned devices are all controlled and operated by the control cabinet 20;
[0047] The outlet of the crushing device is connected with the inlet of the feeding device, the outlet of the feeding device is connected with the inlet of the high-temperature anaerobic pyrolysis device 6, and the gas outlet of the high-temperature anaerobic pyrolysis device 6 is cracked through the The gas pipe 12 is connected to the air inlet of the condensing device 14, the condensing liquid outlet 18 of the condensing device 14 is connected to the condensing liquid inlet 17 of the crysta...
Embodiment 3
[0057] The device of Example 3 is the same as that of Example 2, and the specific process is different.
[0058] The process of the nitrogen trifluoride electrolysis residue resource recovery of the present embodiment comprises the following steps:
[0059] 1) The nitrogen trifluoride electrolysis residue is pulverized by a shredder, and pulverized to particles below 20 mm;
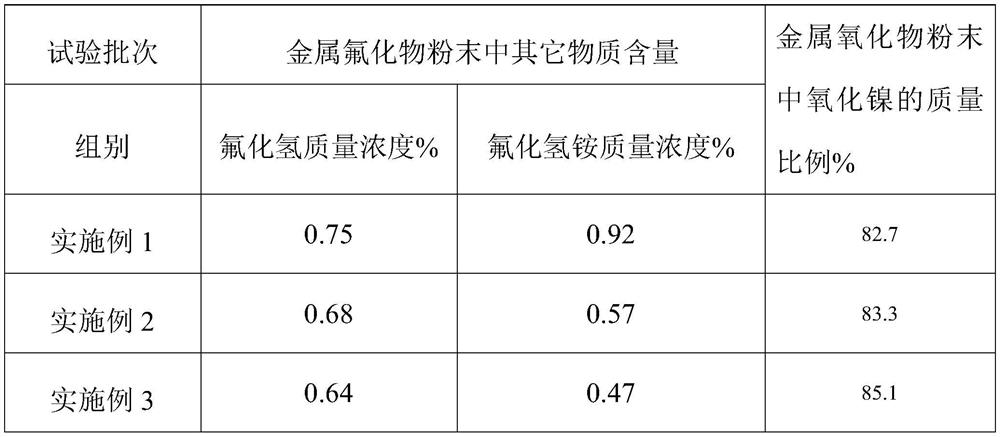
[0060] 2) The pulverized residue is sent to the high-temperature anaerobic pyrolysis device 6 through the feeding device, and high-temperature anaerobic pyrolysis is carried out at 600 ° C to obtain the gas and powder after the pyrolysis; wherein, the powder is metal fluoride powder, The condensation and crystallization adopts water cooling, and the water cooling is normal temperature tap water, and the tap water is cooled by an air-cooled heat exchanger to realize recycling;
[0061] 3) the gas after the pyrolysis is added to the condensing device 14, condensed to obtain condensed liquid and non-condens...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com