Composition for long-acting supplement of arginine and neutralization of acid environment by targeting immune cells and application thereof
A technology of immune cells and compositions, which is applied in the field of preparation of anti-tumor drugs, can solve the problems of weakening proliferation ability, etc., achieve the effects of starvation tumor weakening, enhancing anti-tumor immune response, and good clinical application potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1 A kind of preparation method of composition (microsphere dosage form)
[0042] Composition of the composition (microsphere dosage form):
[0043] L-Arginine 5mg
[0044] CAT-2 siRNA / Lipo8000 complex 7nmol (based on siRNA content)
[0045] Poly(lactic-co-glycolic) acid, 1.5w, 50:50 200mg.
[0046] Dissolve the prescription amount of L-arginine and CAT-2 siRNA / Lipo8000 complex in 200μL of pure water as the inner water phase; the prescription amount of Poly(lactic-co-glycolic)acid is dissolved in 4mL dichloromethane , as the oil phase. The inner water phase was added to the oil phase, vortexed for 30 seconds and then phacoemulsified with a 100W probe for 2 minutes. The obtained W / O emulsion was added to 40 mL of an aqueous solution containing 2% PVA, and after high-speed stirring, it was added to 100 mL of an aqueous solution containing 0.2% PVA, and stirred at low speed overnight to remove dichloromethane and complete the solidification of the microsphere...
Embodiment 2
[0047] Example 2 In vitro release of the composition (microsphere dosage form)
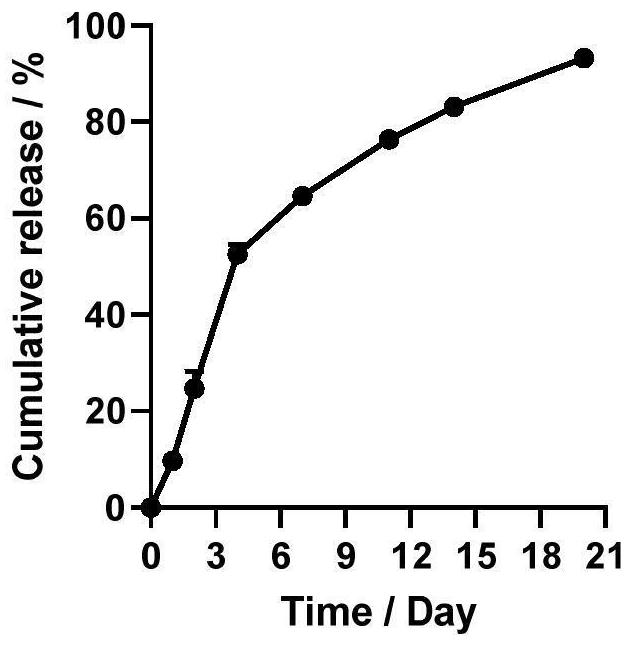
[0048] In this example, on the basis of Example 1, the composition (microsphere dosage form) was taken, resuspended in a PBS solution with a pH of 7.4, placed in a constant temperature shaking box at 37°C, sampled at a set time, and collected after high-speed centrifugation In the supernatant, the L-arginine content was determined by HPLC, and the in vitro release behavior of the composition (microsphere dosage form) was investigated. The release profile showed that the composition (microsphere dosage form) exhibited sustained release capability for up to 21 days in PBS solution with pH 7.4 at 37°C ( figure 1 ).
[0049] The HPLC conditions used were:
[0050] Mobile phase: methanol: 0.01M sodium pentanesulfonate=(10:90), 1 mL / min; detection wavelength: 206 nm; chromatographic column: Agilent ZORBAX C18 column (4.6×250 mm, 5 μm).
Embodiment 3
[0051] Embodiment 3 A kind of preparation method of composition (multivesicular liposome dosage form)
[0052] Composition of the composition (multivesicular liposome dosage form):
[0053]
[0054] Dissolve the prescription amount of L-arg and CAT-2 shRNA plasmid / Lipo8000 complex in 1mL of pure water as the first aqueous phase; the prescription amount of egg yolk lecithin E80, cholesterol and α-tocopherol are dissolved in 2mL of dichloromethane , as the oil phase. The first water phase was added to the oil phase, and the 200W probe was ultrasonically emulsified for 4 minutes to prepare a W / O emulsion. The 5 mg / mL sodium chloride aqueous solution containing 2% sodium dodecyl sulfate was used as the second water phase, the prepared W / O emulsion was added to 10 mL of the second water phase, and the W / O / W was prepared by stirring at a low speed 20 mL of 5 mg / mL sodium chloride solution was added to it, stirred in a water bath at 25°C for 5 hours, and the organic solvent was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com