(diphenyl amino) phenyl flavonoid fluorescent probe for detecting cysteine and preparation method of (diphenyl amino) phenyl flavonoid fluorescent probe
A technology of diphenylamino and cysteine, which can be used in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of complicated operation, expensive equipment, long detection time, etc., and achieve the effect of good application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
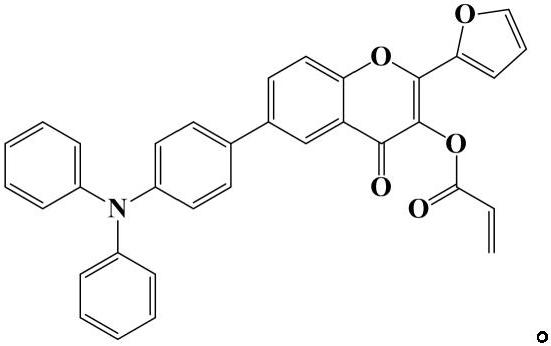
[0031] The preparation of (diphenylamino) phenylflavonoids, the reaction formula is:
[0032]
[0033] Specific steps are as follows:
[0034] 1) Preparation of 4-(diphenylamino)phenyl-2-hydroxyacetophenone:
[0035] 10mmol of 4-bromo-2-hydroxyacetophenone, 12mmol of 4-(diphenylamino)phenylboronic acid, 40mmol of sodium carbonate and 1mmol of tetrakis(triphenylphosphine) palladium were successively added to the dry three-necked flask, and then 50mL of diphenylphosphine was added. Oxane and 15 mL of water were dissolved, heated to 90°C under nitrogen protection and reacted overnight, followed and monitored by TLC method, and the reaction was stopped when the reaction was complete; the reaction solution was distilled under reduced pressure to remove dioxane and water, and then ethyl acetate was added. Ester, washed with saturated brine to neutrality, the organic phase was dried over anhydrous sodium sulfate, filtered and concentrated to obtain 4-(diphenylamino)phenyl-2-hydro...
Embodiment 2
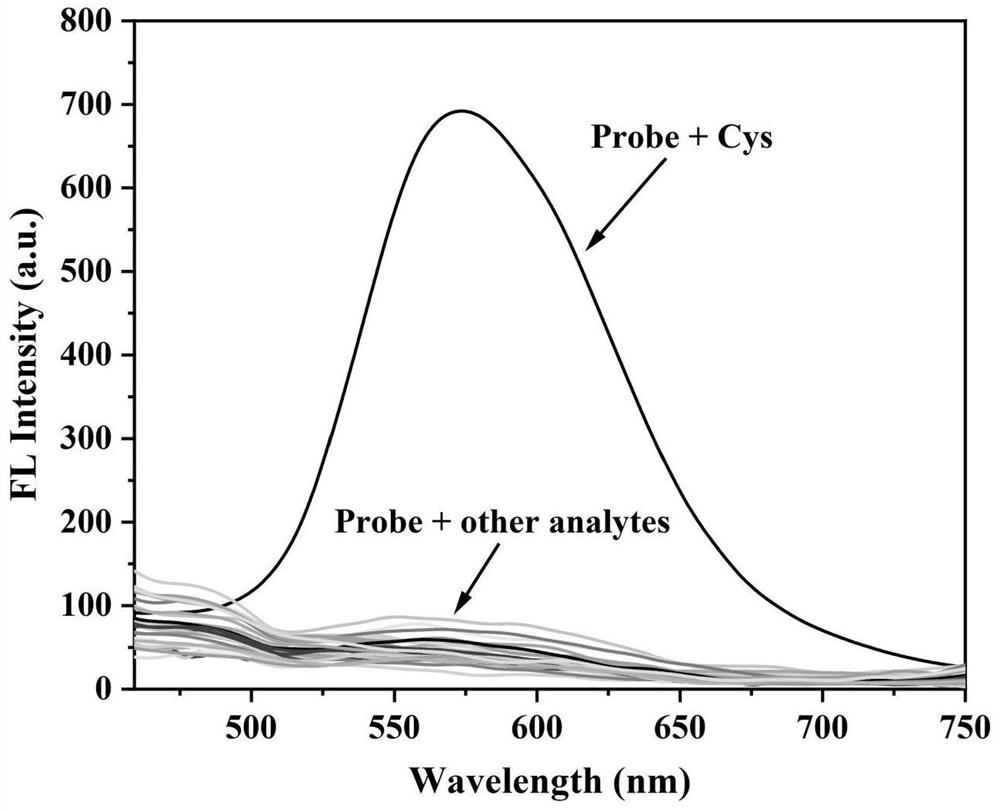
[0041] 6-(4-(Diphenylamino)phenyl)-2-(furan-2-yl)-3-hydroxychromone acrylate and Cys, GSH, Hcy, Gly, Arg, Asp, Ala, His, Lys, Glu, Ser, Val, Tyr, F - , Cl - , Br - , I - , NO 3 - , CO 3 2- , SO 4 2- , HSO 3 - , SO 3 2- , Mg 2+ , Na + , Cu 2+ , Ca 2+ , Ag + , K + 28 different analytes were respectively dissolved in DMSO / PBS (v / v=6 / 4) buffer to prepare a concentration of 1.0×10 -6 Probe solution and concentration of M is 1.0 × 10 -5 28 different analyte solutions of M were used to measure the fluorescence spectrum of the solution using a fluorescence spectrometer, and the results were as follows figure 1 shown. figure 1 It shows that the addition of cysteine makes the fluorescence intensity of the system change from colorless to orange-red under ultraviolet light at 365 nm, and by adding other interfering substances GSH, Hcy, Gly, Arg, Asp, Ala, His, Lys, Glu, Ser, Val, Tyr, F - , Cl - , Br - , I - , NO 3 - , CO 3 2- , SO 4 2- , HSO 3 - , SO ...
Embodiment 3
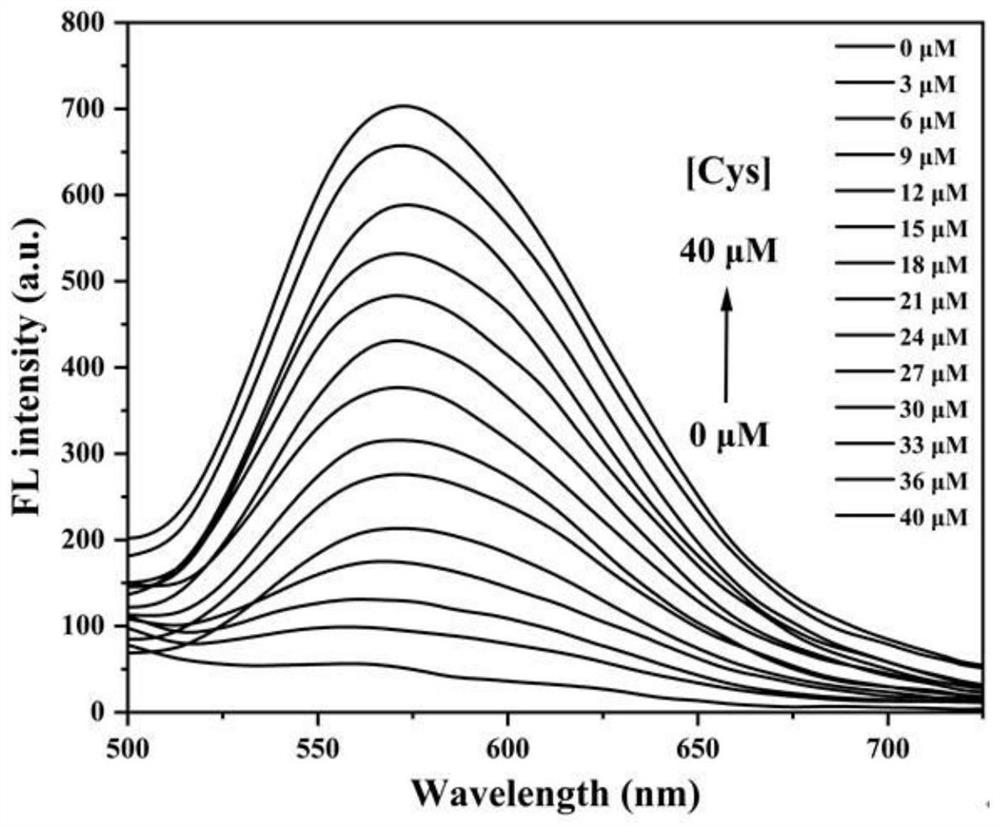
[0043] 6-(4-(Diphenylamino)phenyl)-2-(furan-2-yl)-3-hydroxychromone acrylate was formulated to a concentration of 1.0×10 -5 M in DMSO / PBS (v / v=6 / 4) buffer solution, also dissolve cysteine in DMSO / PBS (v / v=6 / 4) buffer solution to make the concentration of 0, 3, 6, 9 , 12, 15, 18, 21, 24, 27, 30, 33, 36, 40 μM solutions. The effect of different concentrations of cysteine on the fluorescence spectrum of 6-(4-(diphenylamino)phenyl)-2-(furan-2-yl)-3-hydroxychromone acrylate by titration , the result is figure 2 shown. figure 2 It shows that with the continuous increase of cysteine concentration in the system, the fluorescence intensity at 575nm gradually increases, which indicates that the compound can detect cysteine sensitively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com