Application of glycosyl transferase mutant in directional synthesis of non-natural ginsenoside
A technology of glycosyltransferase and ginsenoside, which is applied in the application field of glycosyltransferase BcGT1 mutant in the enzymatic preparation of unnatural ginsenoside, which can solve the problems of low substrate concentration, poor reaction selectivity and expression of glycosyltransferase To achieve the effects of high selectivity, increased yield, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: This example illustrates the cloning of the glycosyltransferase BcGT1
[0031] Microbial strain genome extraction: take the standard strain: Bacillus cereus ATCC14579 as the starting strain, and extract the target genome with reference to the extraction method of the microbial genome extraction kit (TaKaRa MiniBEST Bacteria Genomic DNA Extraction Kit Ver.3.0). Store at -20°C for short-term use.
[0032] The cloning primers were designed with reference to the target glycosyltransferase gene in the genome information of Bacillus cereus NC7401 in the NCBI database as a template. Using the extracted Bacillus cereus genome as a template, the target glycosyltransferase gene fragment BcGT1 was obtained by PCR amplification with the corresponding cloning primers. After the target glycosyltransferase gene fragment BcGT1 obtained by PCR amplification was verified by agarose gel electrophoresis, the target gene fragment was purified and recovered according to the AxyPr...
Embodiment 2
[0033] Example 2: This example illustrates the construction of a glycosyltransferase gene expression vector
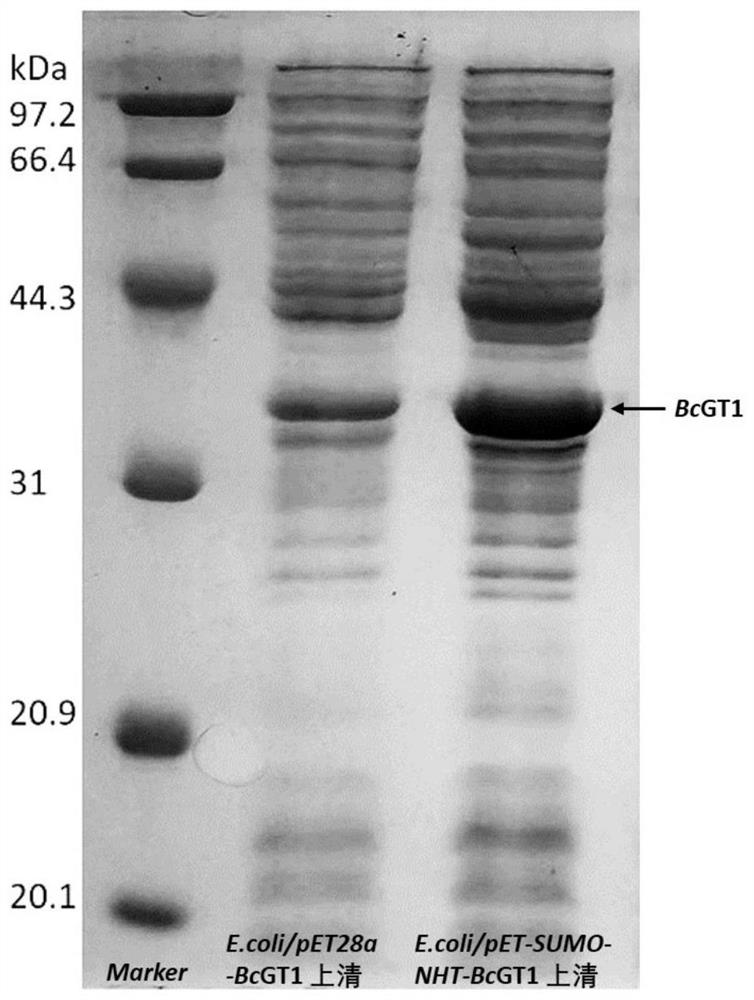
[0034] In order to achieve the high-efficiency expression of glycosyltransferase, in the construction of the gene expression vector, the SUMO solubilization tag and the intein NHT intein NHT gene sequence are designed to be added as SEQ ID NO: 3, and the SUMO gene sequence is SEQ ID NO: 4 .
[0035] SUMO can be used as a fusion tag and molecular chaperone for recombinant protein expression, which not only can further improve the expression of fusion protein, but also has the functions of resisting protease hydrolysis, promoting the correct folding of target protein, and improving the solubility of recombinant protein. Intein NHT exists in the same open reading frame (ORF) as the host protein gene, and is transcribed and translated synchronously with the host protein gene. After translation to form a protein precursor, the intein NHT self-splicing and fusion Protein, t...
Embodiment 3
[0045] Example 3: This example illustrates site-directed mutagenesis of the glycosyltransferase BcGT1
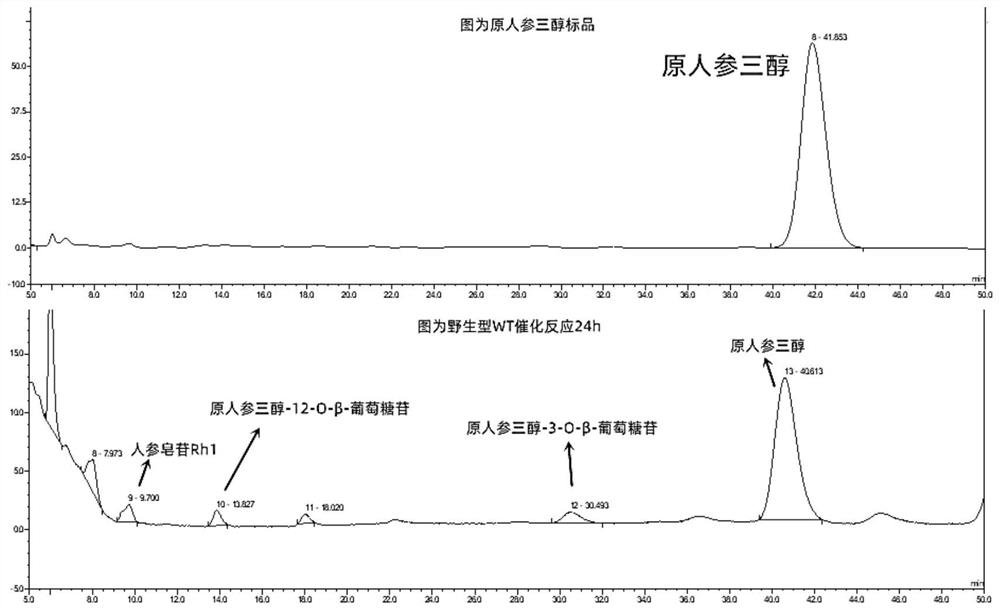
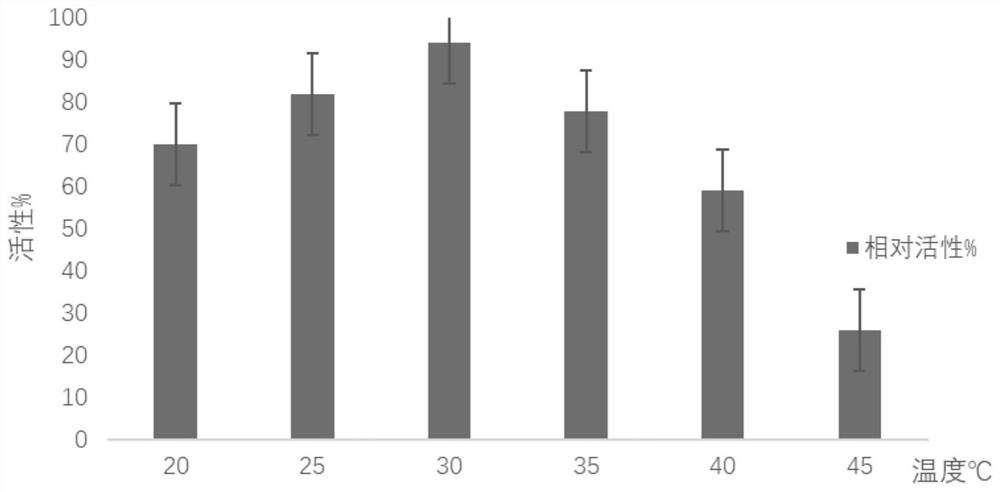
[0046] The three-dimensional structural model of the glycosyltransferase BcGT1 was established with the swiss-model online server, and hotspotwizard online software was used to scan and predict the hot spots. The predicted amino acid residues around the active center were mutated to alanine, and the glycosyltransferase BcGT1 was mutated to alanine. The amino acid sites are P18A, L59A, S60A, I64A, H80A, E86A, N108A, F133A, E142A, N181A, T325A, G326A, respectively. The mutants of glycosyltransferase BcGT1 were respectively induced and expressed, and the mutants were prepared. The wild-type glycosyltransferase BcGT1 or its mutants were used to catalyze the synthesis of unnatural ginsenosides, the products were detected by high performance liquid chromatography, and the conversion rate and product selectivity of the mutant catalyzed substrates were calculated. Two mutants P18A ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com