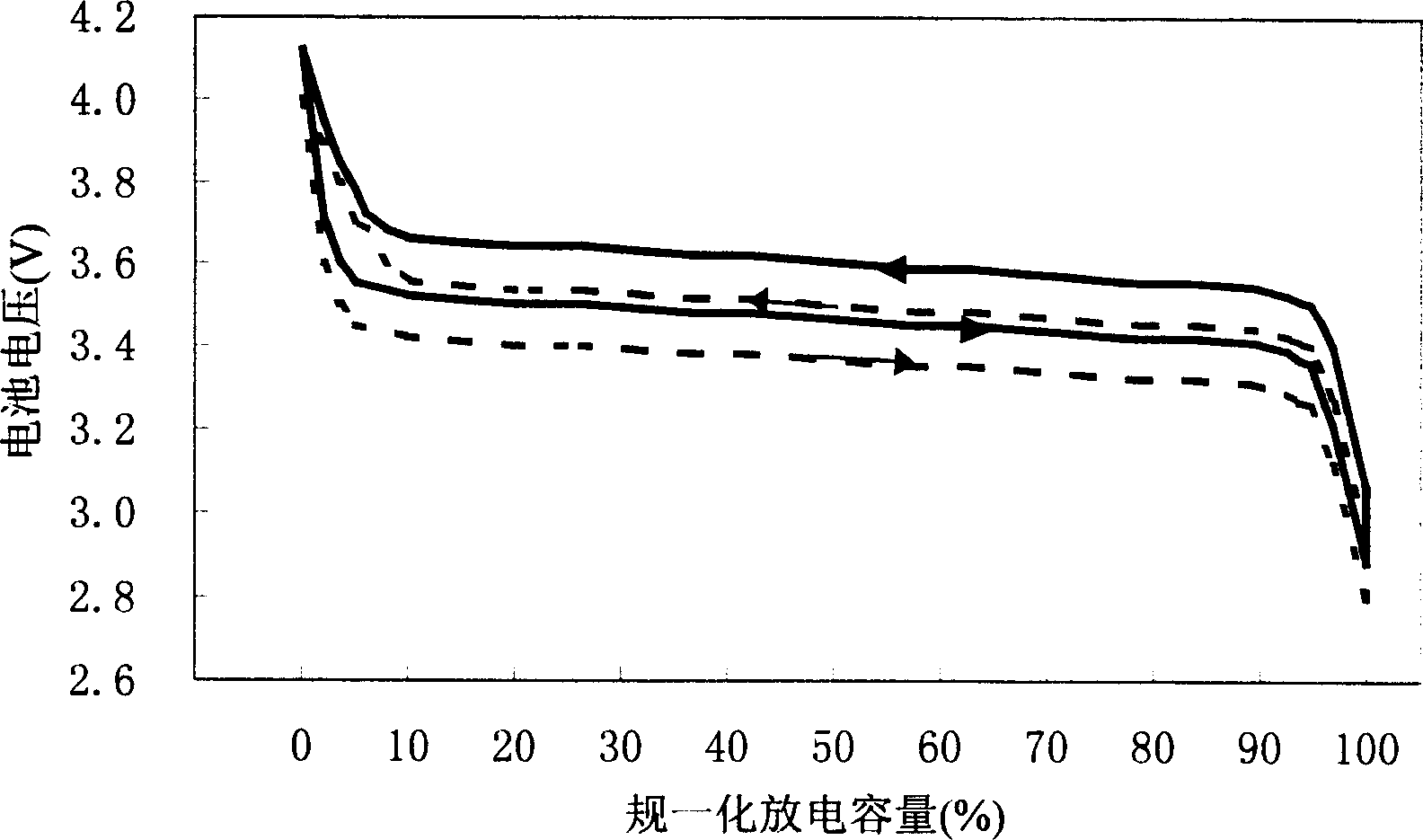
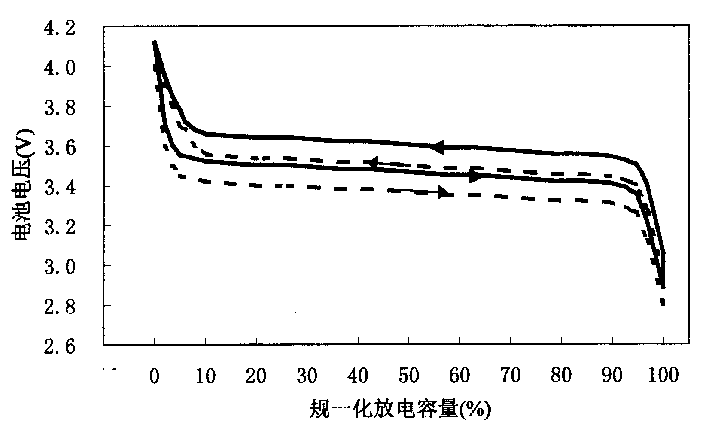
Lithium cell positive electrode materials and preparing method thereof
A technology for positive electrode materials and lithium batteries, applied in electrode manufacturing, battery electrodes, positive electrodes, etc., can solve problems that affect the energy density of materials, are not suitable for large-scale industrial production, and the method of adding trace elements is complicated.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1: the polycrystalline solid powder of the magnesium-doped lithium iron phosphate LixMg1-xFePO of preparation high conductivity
[0063] In the first step, 356 grams of iron thioninate Fe(NH4)2(SO4)2·6H2O and 115 grams of ammonium phosphate NH4H2PO4 were dissolved in 2000 grams of deionized water while stirring. Oxygen was introduced into the aqueous solution, and the temperature was raised to 95° C. for 10 hours. In oxidizing atmosphere and aqueous solution, iron thionate Fe(NH 4 ) 2 (SO 4 ) 2 ·6H 2 O and ammonium phosphate NH 4 h 2 PO 4 Reaction to form precipitate, amorphous phase FePO 4 .
[0064] In the second step, add 24 grams of lithium hydroxide to the above-mentioned semi-finished FePO aqueous solution, and keep it at a temperature of 95° C. for 3 hours. LixFePO4 in an unsaturated state is formed after chemical diffusion in the liquid. The product is washed with water to remove the remaining reactants, and finally vacuum-dried to make uns...
Embodiment 2
[0067] Example 2: Preparation of polycrystalline solid powder of non-stoichiometric lithium iron phosphate LiFePO4-y with high conductivity
[0068] In the first step, 174 grams of iron acetate (CH3COO) 2 Fe, 115 grams of ammonium phosphate NH4H2PO4 and 20 grams of carbon gel were successively added to 1000 grams of ethanol under stirring to form a sol emulsion.
[0069] In the second step, 66 grams of lithium acetate CH3COOLi are dissolved in the above-mentioned sol emulsion under stirring.
[0070] In the third step, the sol solution was transferred to an alumina ceramic crucible, and heated at 150° C. for 2 hours in a sealed furnace. Then, at 10 -2 Under low Torr vacuum, slowly heat to 500°C for 5 hours. Part of the oxygen element reacts with the carbon gel to generate CO and discharge CO2 under the condition of vacuum heating. Finally, under atmospheric conditions, the temperature was raised to 700° C. and kept for 5 hours to complete the polycrystallization reaction o...
Embodiment 3
[0072] Example 3: Preparation of supercharged magnesium-doped lithium iron manganese phosphate Li x Mg 1-x Fe z mn 1-z PO 4
[0073] The first step: 180 grams of iron thioninate Fe (NH 4 ) 2 (SO 4 ) 2·6H 2 O, 178 g Mn(NH 4 ) 2 (SO 4 ) 2 ·6H 2 O with 115 g ammonium phosphate NH 4 h 2 PO 4 Dissolve in 2000 g deionized water with stirring. Oxygen was introduced into the aqueous solution, and the temperature was raised to 95° C. for 10 hours. In oxidizing atmosphere and aqueous solution, iron thionate Fe(NH 4 ) 2 (SO 4 ) 2 ·6H 2 O, Mn(NH 4 ) 2 (SO 4 ) 2 ·6H 2 O and ammonium phosphate NH 4 h 2 PO 4 Reaction to form precipitates, amorphous phase Fe 0.5 mn 0.5 PO 4 .
[0074] In the second step, in the above-mentioned semi-finished Fe 0.5 mn 0.5 PO 4 Add 24 grams of lithium hydroxide to the mixture and keep it at 95°C for 3 hours. Li in an unsaturated state after chemical diffusion in a liquid x FePO 4 . The product is washed with water to rem...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com