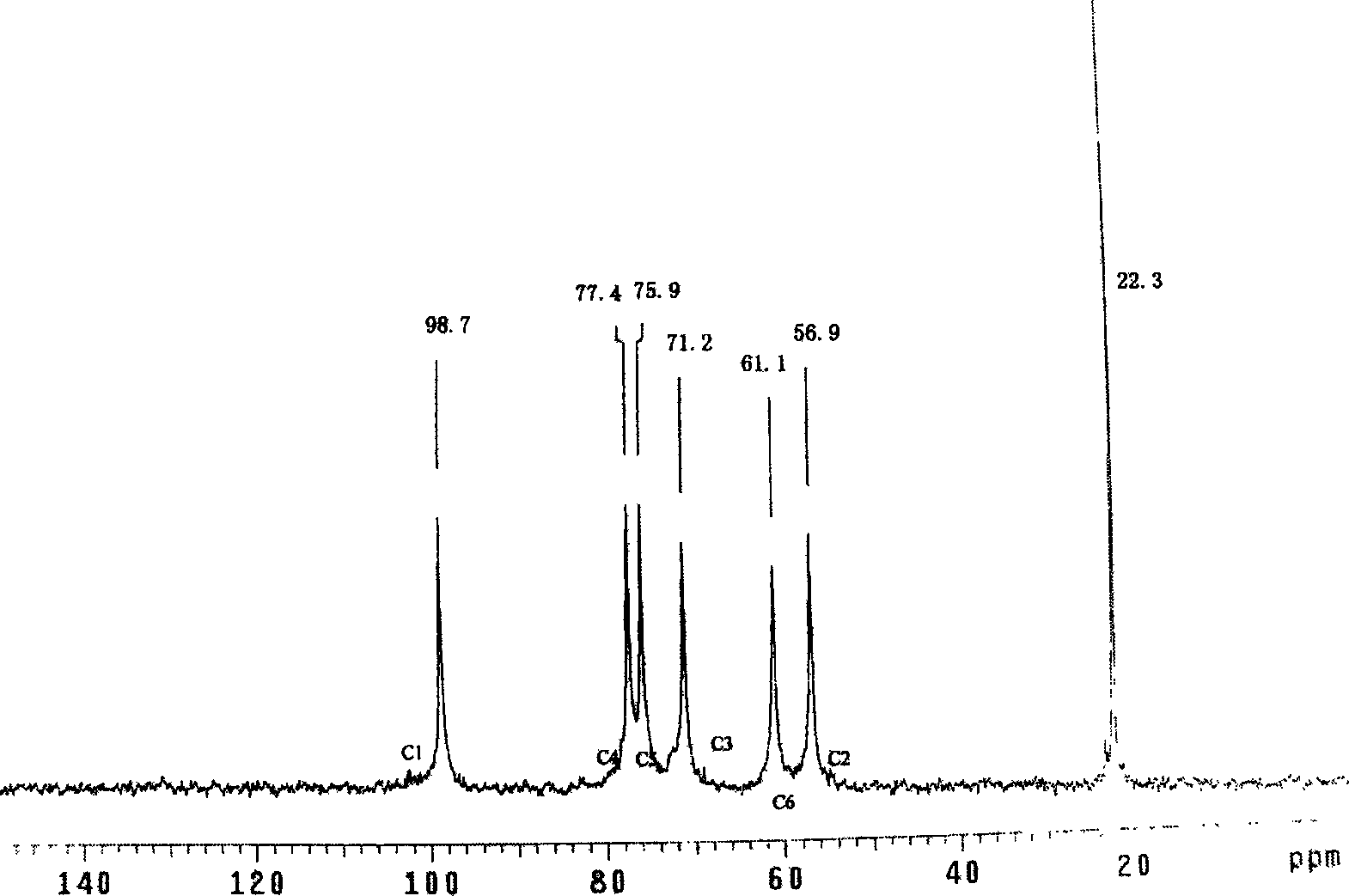
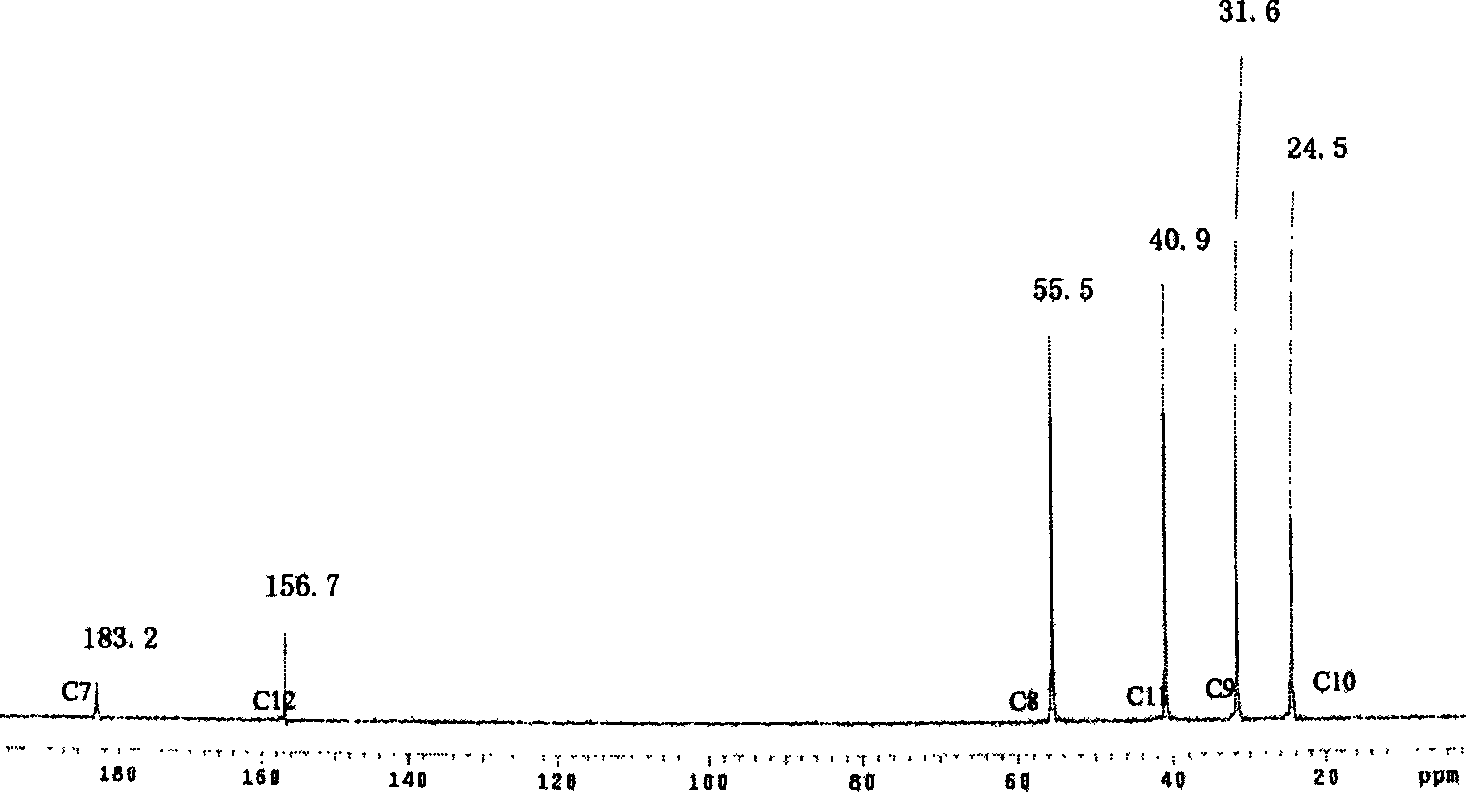
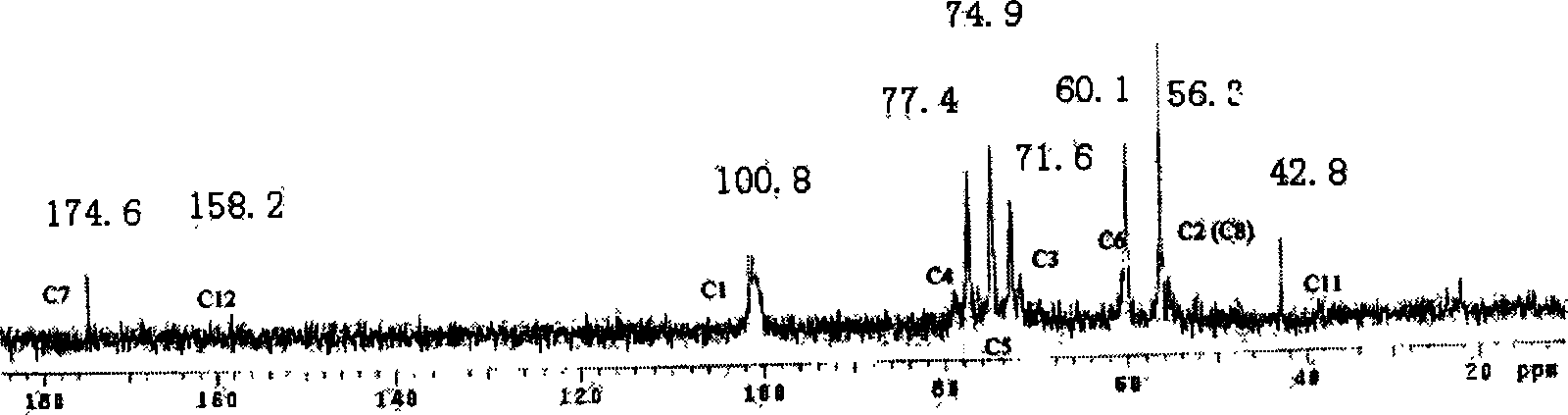
Method for perparing anticoagulant composed of chitosan-arginine
A technology of arginine and chitosan, which is applied in the field of preparation of blood-compatible materials, can solve the problems of unsatisfactory anti-coagulation properties of anti-coagulation materials, limited application of cytotoxicity, etc., and achieves improved anti-coagulation performance, The effect of reducing cytotoxicity and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Weigh 13 grams of sodium periodate, dissolve it in 100 grams of deionized water, add 10 grams of glucose, place in a dark box, add 10 μl of hydrochloric acid dropwise to adjust the pH to acidity, react at room temperature for 12 hours, add barium acetate and sodium sulfate in turn, React with unreacted excess sodium periodate and barium ions respectively, centrifuge to remove the precipitate, and freeze-dry the supernatant to obtain oxidized glucuronide.
[0018] Get 2.0 grams of chitosan with a degree of deacetylation of 80% and a molecular weight of 5000, add it to the TEMED solution that is adjusted to a pH of 4.8 with HCl, add 0.0906 grams of 1-ethyl-3-(3-dimethylaminopropyl) Carbodiimide and 0.0546 g of N-hydroxy-succinimide were stirred evenly, then 0.082 g of arginine was added, reacted at room temperature for 8 hours, dialyzed in deionized water for 5 days, and dried at room temperature to obtain the final product. Dissolve 200 mg of refined chitosan-arginine co...
Embodiment 2
[0024] Get 2.0 grams of chitosan with a degree of deacetylation of 80% and a molecular weight of 5000, add it to the TEMED solution that is adjusted to a pH of 4.8 with HCl, add 0.3624 grams of 1-ethyl-3-(3-dimethylaminopropyl) Carbodiimide and 0.2184 g of N-hydroxy-succinimide were stirred evenly, then 0.328 g of arginine was added, reacted at room temperature for 8 hours, dialyzed in deionized water for 5 days, and dried at room temperature to obtain the final product. Dissolve 200 mg of refined chitosan-arginine conjugate in deionized water, add 10 mg of glucosin oxide, stir evenly, pour it into a plastic petri dish with a smooth surface, and react in a vacuum oven at 37 °C for 24 hours, and finally A crosslinked film was obtained. Cut the film sample into 5mm×10mm rectangular pieces, soak it in 0.9% normal saline for 24 hours, and use it as a sample for anticoagulant performance testing. Deacetylation degree 80%, and molecular weight 50,000, deacetylation degree 80% chito...
Embodiment 3
[0029] Get 2.0 grams of chitosan with a deacetylation degree of 80% and a molecular weight of 50,000, join in the TEMED / HCl buffer solution of pH 4.8, add 0.5436 grams of 1-ethyl-3-(3-dimethylaminopropyl) carbon Diimine and 0.3276 g of N-hydroxy-succinimide were stirred evenly, then 0.492 g of arginine was added, reacted at room temperature for 8 hours, neutralized, dialyzed in deionized water for 5 days, and freeze-dried to obtain the final product. Dissolve 200 mg of refined chitosan-arginine conjugate in deionized water, add 10 mg of glucosin oxide, stir evenly, pour it into a plastic petri dish with a smooth surface, and react in a vacuum oven at 37 °C for 24 hours, and finally A crosslinked film was obtained. The film sample was cut into 5 mm×10 mm rectangular pieces, soaked in 0.9% saline for 24 h, and used as a sample for anticoagulant performance testing; the obtained sample was designated as CS1-ArgC3-1. According to the same preparation steps and conditions, samples...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com