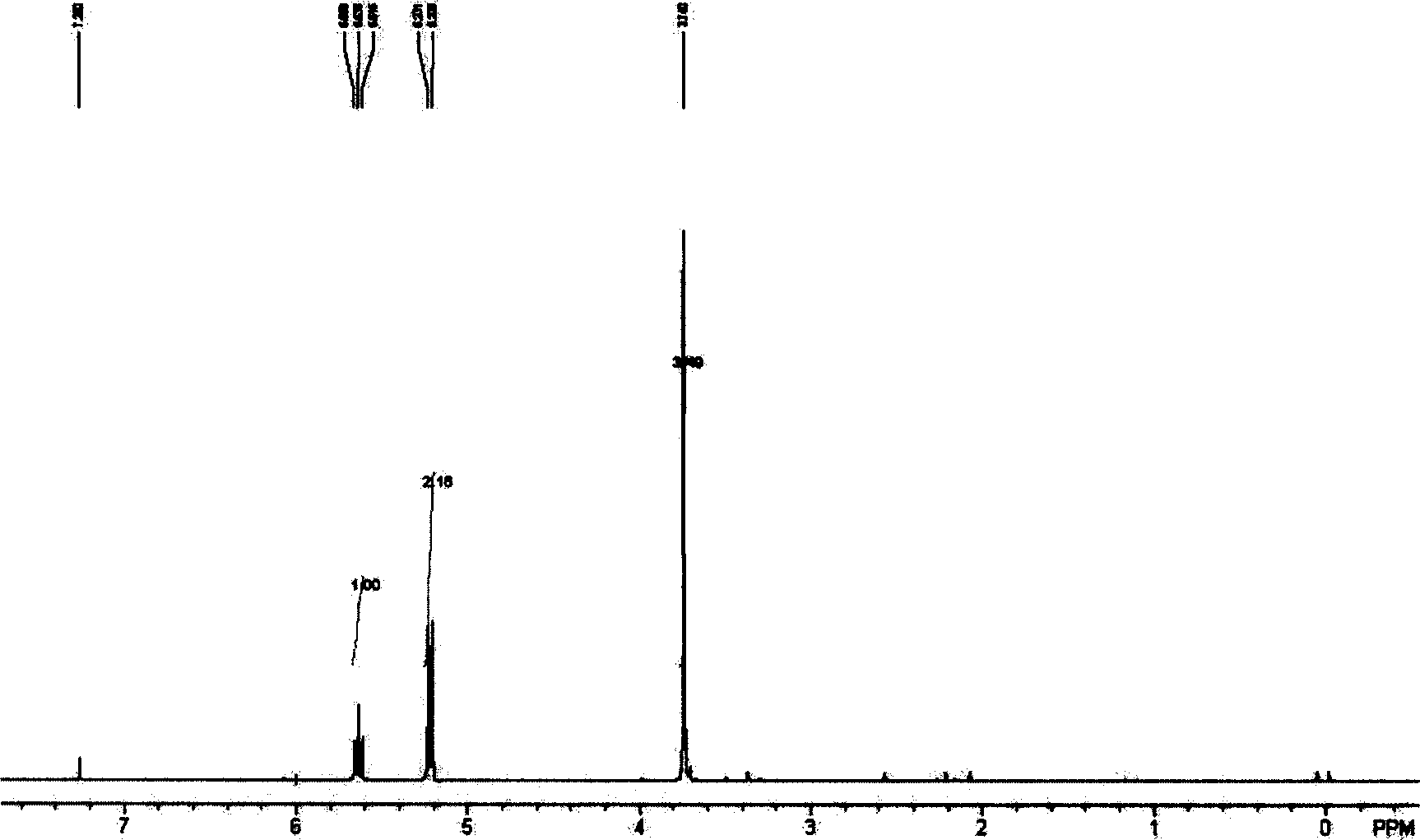
Production of 1,2-diene methyl butyrate
A technology for the production of methyl diene butyrate, which is applied in 1, can solve the problems of increased production cost, large amount of solvent, and high price, and achieve the effects of improved production efficiency, simple reaction conditions, and short reaction cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Dissolve 1000 g (3.82 mol) of triphenylphosphine in 3000 ml of benzene and place it in a 5 L reaction flask. At room temperature, 362 ml (3.82 mol) of methyl α-bromoacetate was slowly added dropwise, and reacted overnight at room temperature, and a large amount of white solid precipitated out. After filtration, the solid was washed with ether and dissolved in warm water. After complete dissolution, basify with 2N aqueous sodium hydroxide solution, a large amount of white solids precipitate out of the solution, filter after cooling, wash the solids with water, and dry in the air. 1223 g of white solid methoxycarbonylmethylene triphenylphosphine were obtained, yield: 95.9%.
[0022] Dissolve 334 grams (1.0 mol) of methoxycarbonylmethylene triphenylphosphine in 1100 ml of dichloromethane and 169 ml of triethylamine (1.2 mol), and add 86 ml (1.2 mol) of acetyl chloride and 50 ml of dichloromethane dropwise under reduced pressure. The mixed solution of methane was added in...
Embodiment 2
[0025] The preparation method of methoxycarbonylmethylene triphenylphosphine is the same as Example 1.
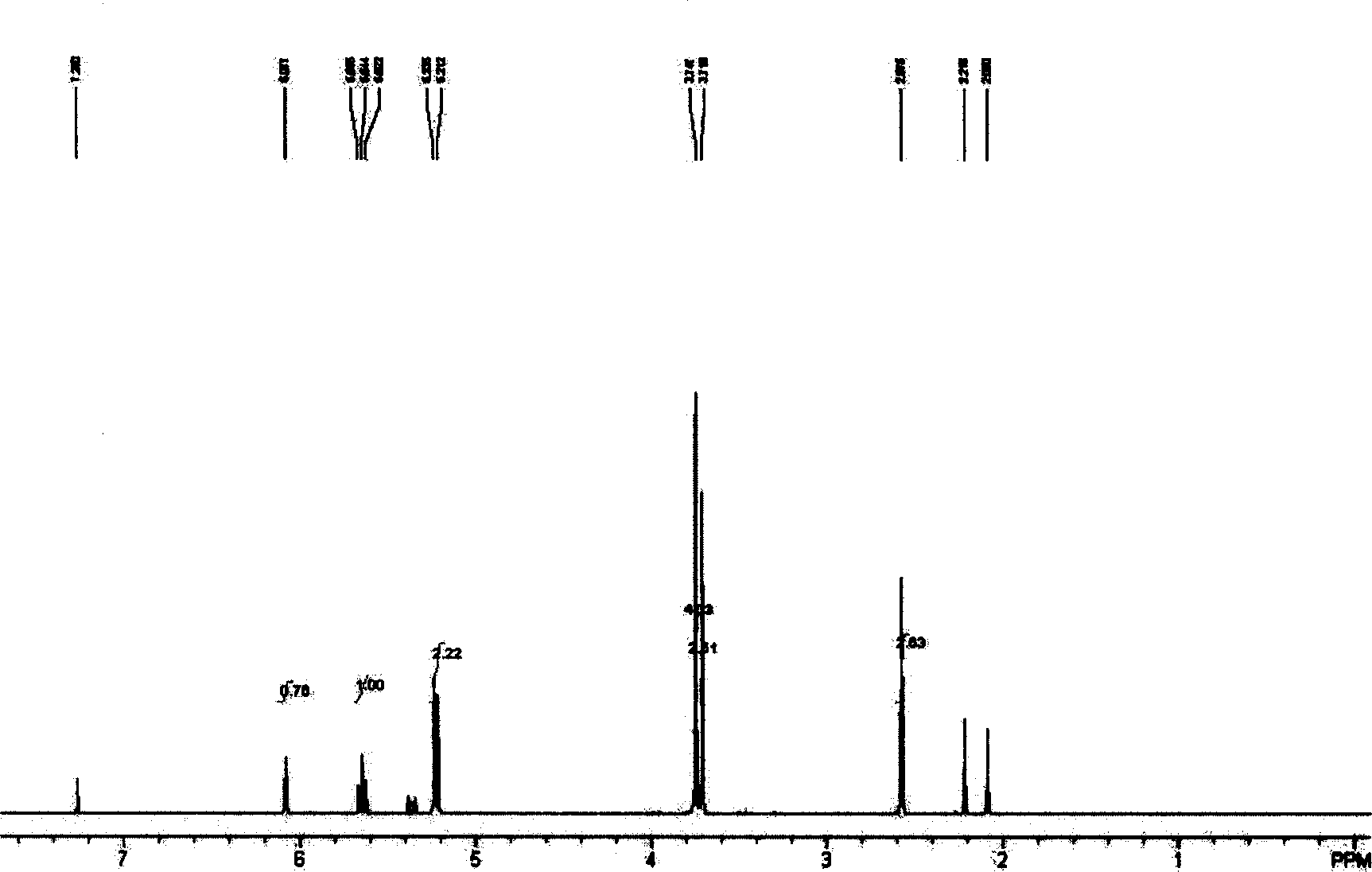
[0026] Dissolve 167 grams (0.5 mol) of methoxycarbonylmethylene triphenylphosphine in 700 ml of dichloromethane and 140 ml of triethylamine (1.0 mol), and add 37 ml (0.5 mol) of acetyl chloride and 150 ml of dichloromethane dropwise at room temperature After the addition, the reaction was continued at room temperature for 10 hours. After the reaction stopped, the oil pump decompressed to extract the product solution, the obtained solution was concentrated, and the water pump decompressed to distill the product to obtain 20 g of colorless liquid with a yield of 41%. Additional products 1 HNMR spectrum, figure 2 It shows that while containing the product 1,2-diene butanoic acid methyl ester, another 2-chloro-2-butenoic acid methyl ester, acetic acid and acetic anhydride impurities are generated.
Embodiment 3
[0028] The preparation method of methoxycarbonylmethylene triphenylphosphine is the same as Example 1.
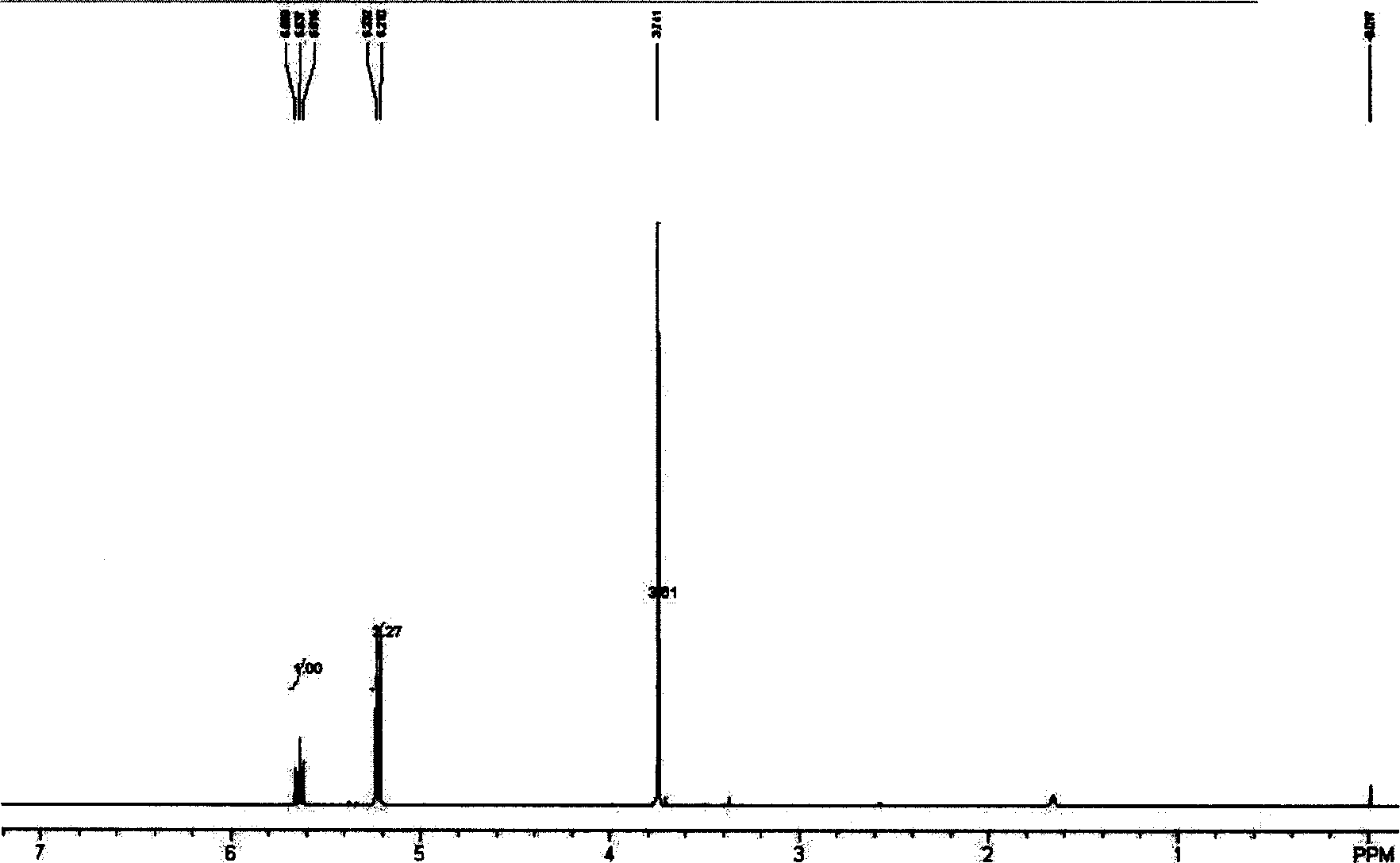
[0029]3.4 grams (0.01mol) of methoxycarbonyl methylene triphenylphosphine are dissolved in the dichloromethane that 40ml phosphorus pentoxide is processed, and the triethylamine ( 0.02mol) and 10ml of dichloromethane mixed solution was stirred for 10 minutes, and then a mixed solution of redistilled acetyl chloride 1.1ml (0.01mol) and 10ml of dichloromethane was added dropwise. After the addition was complete, the reaction was continued at room temperature for 0.5 hours. After the reaction stopped, the obtained reaction mother liquor was concentrated, washed with petroleum ether for 4 times, filtered and combined to concentrate the organic layer, and the product was distilled off under reduced pressure by a water pump to obtain 0.5 g of light yellow liquid with a yield of 51%. Additional products 1 HNMR spectrum, see image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com