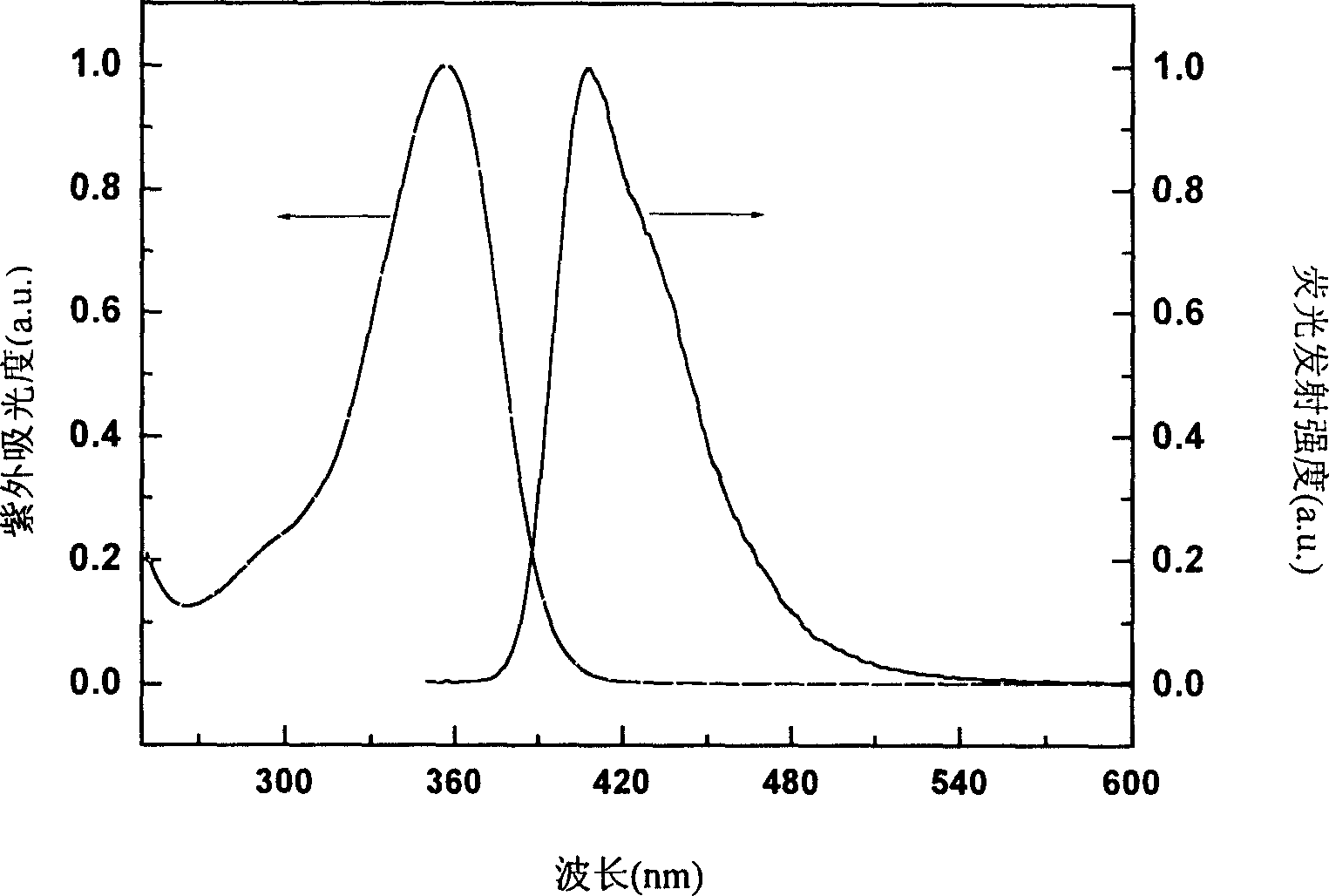
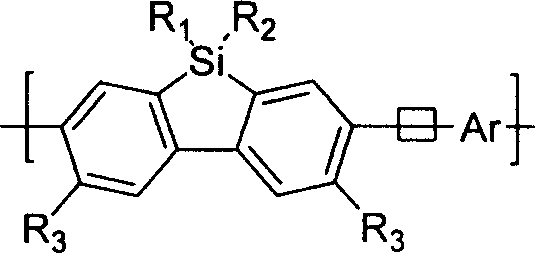
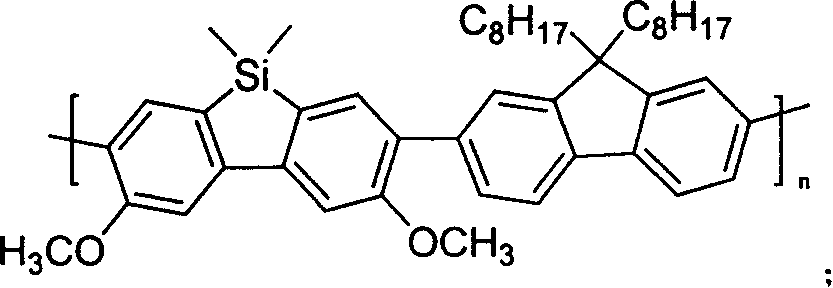
Novel polyfluorene derivative-polysilicofluorene and method for preparation thereof
A technology of silfluorene and compounds, which is applied in the field of silfluorene polymers and their synthesis, can solve problems such as lack of literature reports and synthesis difficulties, and achieve good application prospects, excellent luminous performance and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Synthesis of poly[(3,6-dimethoxy-9,9'-dimethyl-9-silafluorene)-co-alt-(9,9'-dioctylfluorene)]3,3'- Dimethoxybenzidine was diazotized in HBr, and brominated under the catalysis of CuBr. After 12 hours of reaction, CH 2 Cl 2Extract and spin dry the solvent to obtain crude product. The crude product was separated by column chromatography to obtain the intermediate 4,4'-dibromo-3,3'-dimethoxybiphenyl (1) as a yellow powdery solid. Intermediate (1) in glacial acetic acid with I 2 / KIO 3 Add iodine, react overnight, add 2 times water and filter to obtain a yellow solid product precipitate. The crude product was separated by column chromatography to obtain the intermediate 4,4'-dibromo-6,6'-diiodo-3,3'-dimethoxybiphenyl (2) as a light yellow powdery solid. The intermediate (2) was reacted with n-butyllithium at -100°C for half an hour, then added dichlorodimethylsilane, reacted overnight at room temperature, extracted with ether, and separated by a column to obtain a whit...
Embodiment 2
[0046] Synthesis of poly[(3,6-dimethoxy-9,9'-diphenyl-9-silafluorene)-co-alt-(2,5-dihexyloxybenzenediene)]
[0047] The intermediate (2) in Example 1 reacted with n-butyllithium at -100°C for half an hour, then added dichlorodiphenylsilane, reacted overnight at room temperature, extracted with ether, separated by column, and recrystallized several times Afterwards, a white solid 2,7-dibromo-3,6-dimethoxy-9,9'-diphenyl-9-silafluorene (denoted as A2) was obtained.
[0048] In the ethanol suspension of KOH, add the ethanol solution of hydroquinone dropwise, then add bromide n-hexane dropwise, and after reflux for 24 hours, dihexyloxybenzene is obtained, and then catalyzed with liquid bromine in the chloroform solution with ferric trichloride The reaction is to obtain 1,4-dibromo-2,5-dihexyloxybenzene, which is then catalyzed by triphenylphosphorous palladium in toluene solution to react with tributylvinyltin to obtain 1,4-diene-2, 5-Dihexyloxybenzene (marked as B2)
[0049] Equ...
Embodiment 3
[0051] Synthesis of poly[(3,6-dimethyl-9-methyl-9'-phenyl-9-silicon)-co-alt-(2,5-dihexyloxybenzenediyne)]
[0052] 3,3'-Dimethylbenzidine was diazotized in HBr, and brominated under the catalysis of CuBr. After 12 hours of reaction, CH 2 Cl 2 Extract and spin dry the solvent to obtain crude product. The crude product was separated by column chromatography to obtain the intermediate yellow powdery solid 4,4'-dibromo-3,3'-dimethylbiphenyl (1'). Intermediate (1') was treated with I in glacial acetic acid 2 / KIO 3 Add iodine, react overnight, add 2 times water and filter to obtain a yellow solid product precipitate. The crude product was separated by column chromatography to obtain the intermediate 4,4'-dibromo-6,6'-diiodo-3,3'-dimethylbiphenyl (2') as a light yellow powdery solid. The intermediate (2') was reacted with n-butyllithium at -100°C for half an hour, then added dichloromethylphenylsilane, reacted overnight at room temperature, extracted with ether, and obtained a ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| luminance | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com