New method for preparing 2-deoxy-D-glucose
A technique of glucose and a new method, which is applied in the fields of anti-cancer, drug preparation, and chemical preparation of 2-deoxy-D-glucose, an antibacterial drug raw material drug, can solve the problems of 2-deoxy-D-glucose racemization, 2-deoxy - Low yield of D-glucose, complex production process and other problems, to achieve the effect of low price, novel design, reasonable and feasible, and strong antiviral effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
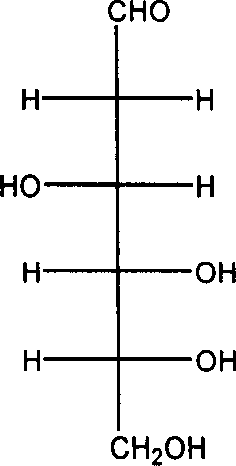
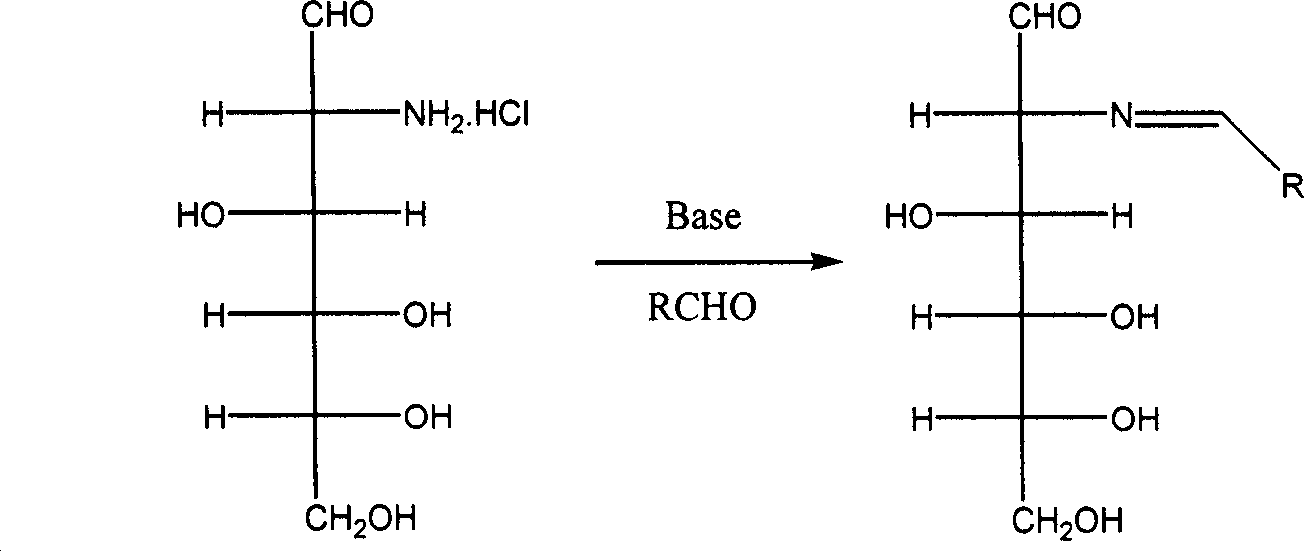
[0052] (1) Dissolve 4.0g of sodium hydroxide in 100mL of water, cool in an ice-salt bath to 0°C, add 10.8g of 2-deoxy-2-amino-D-glucose hydrochloride under stirring, the solid is completely dissolved, and a light yellow color is obtained Clear and transparent solution. 5.3 g of benzaldehyde was added at 0°C, and a large amount of white solid 2-deoxy-2-benzylideneamino-D-glucose was formed. After 2 hours of reaction, the white solid 2-deoxy-2-benzylideneamino-D-glucose was washed with water, filtered, and the obtained filter cake was dried under reduced pressure at 50°C to obtain a white powder of 2-deoxy-2- 12.1 g of benzylidene amino-D-glucose was directly used in the acetylation reaction.
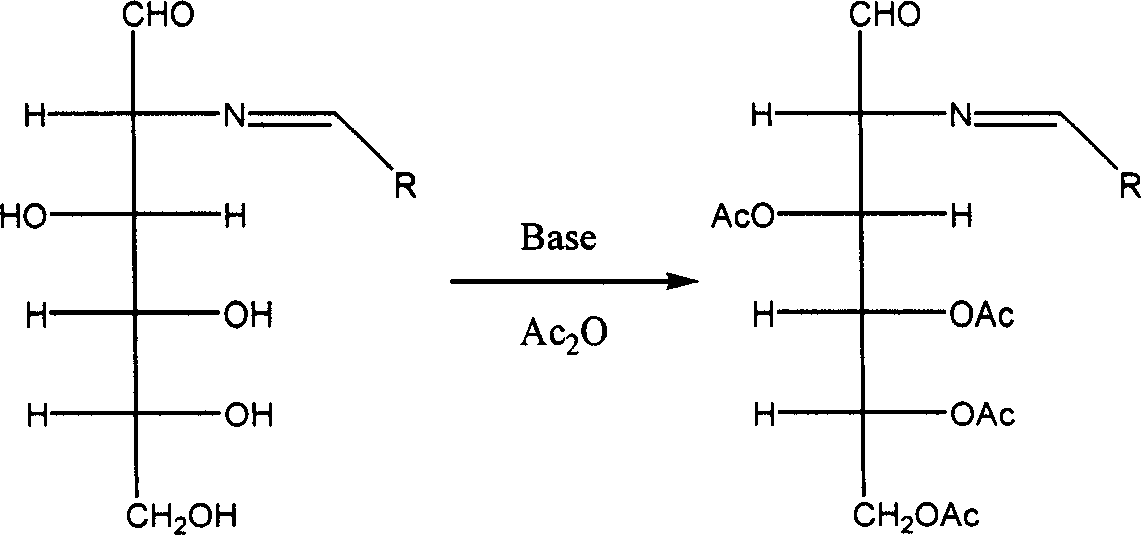
[0053] (2) To 100 mL of acetic anhydride, add 10.7 g of 2-deoxy-2-benzylideneamino-D-glucose, the product of step (3), and 10.8 g of sodium acetate, and react at room temperature for 2 hours. The solvent was evaporated under reduced pressure below 30°C, the obtained residue was added to...
Embodiment 2
[0059] Under the condition that the reaction temperature, the concentration of the reactant, and the feeding method are unchanged, the feeding of the reactants in each step in Example 1 is scaled up by 5 times, and the yield and total yield of each step are basically the same as in Example 1.
Embodiment 3
[0061] Adopt the same operating process as Example 1, in step (1) replace sodium hydroxide 4.0g, 100ml ethanol with potassium hydroxide 7.0g to replace 100ml water, p-methyloxybenzaldehyde 6.5g replace benzaldehyde 5.3g, react 1 hour, the reaction temperature is 50 ℃, in step (2) with triethylamine 26.8g instead of sodium acetate 10.8g, acetic acid instead of acetic anhydride as reaction medium, react for 4 hours, reaction temperature 20 ℃, two-step yield 68.2% , in step (3), sulfuric acid-aqueous solution replaces hydrochloric acid-methanol solution, and reaction temperature is 10 ℃; In step (4), KNO 2 instead of NaNO 2 , replace 90ml tetrahydrofuran / ethanol solution with 100ml water / methanol solution in step (5), replace 1.3g sodium borohydride with 1.8g potassium borohydride, react for 2 hours, yield 95.1%; Replace with methanol in step (6) Ethanol was used as the extraction solvent, the reaction temperature was 30°C, and the mass yield was 43.2%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com