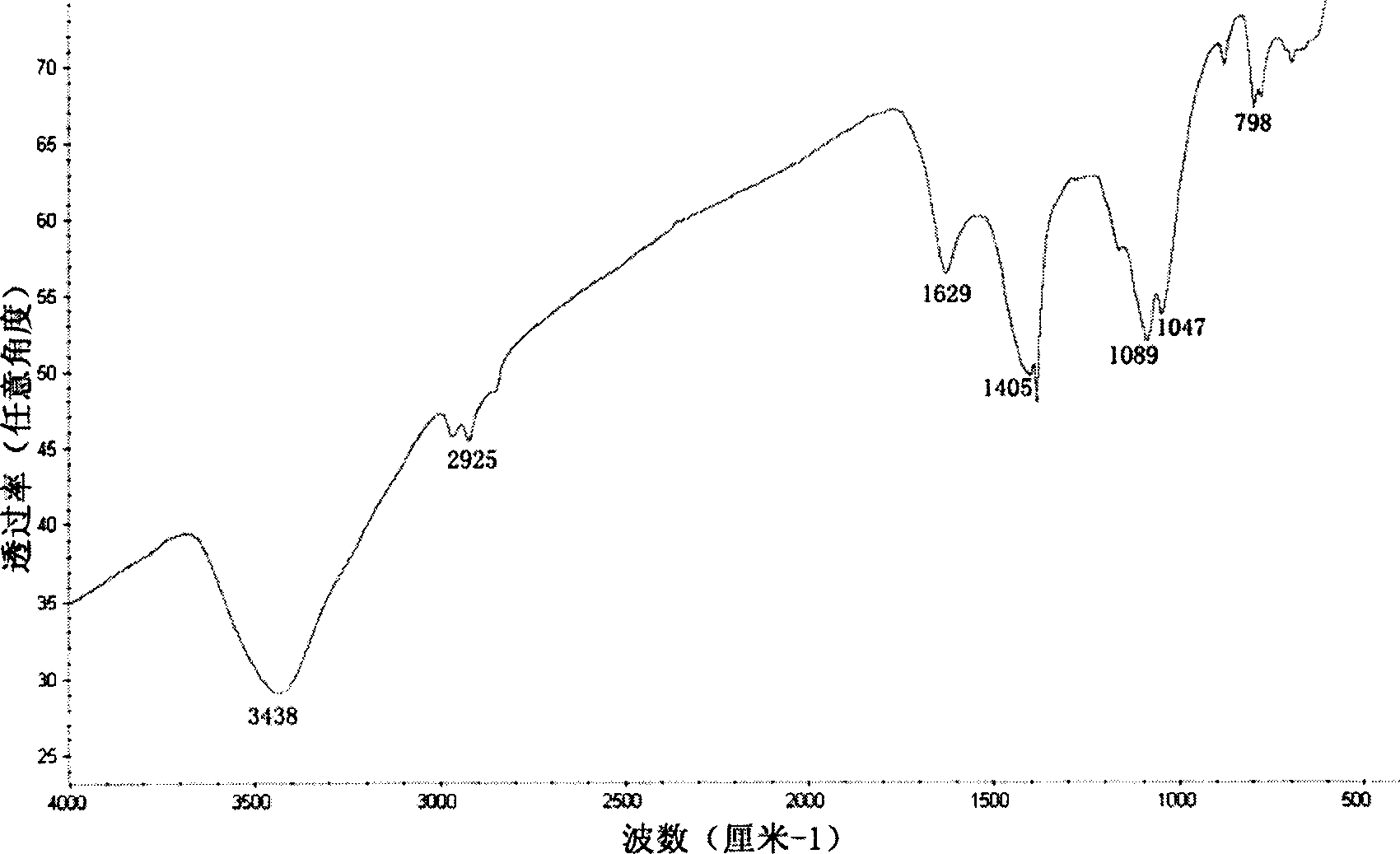
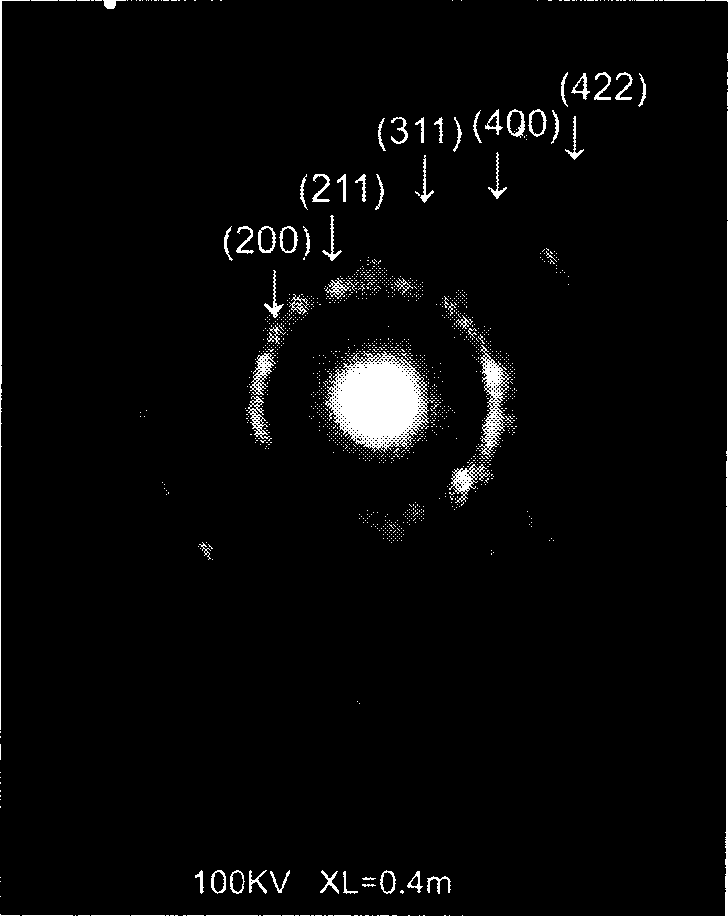
Controllable hydrothermal constant pressure synthesis method for preparation of boron-carbon-nitrogen material
A synthesis method, boron-carbon-nitrogen technology, applied in the direction of nitrogen compounds, chemical instruments and methods, nitrogen and non-metallic compounds, etc., can solve the problem of unsatisfactory uniformity of the reaction system, difficulties in the dissolution and transportation of inorganic substances, poor solubility, etc. problem, to achieve the effect of good phase purity, inhibition of phase reversal, and high solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Firstly, nitrogen gas is bubbled into the deionized water to eliminate dissolved oxygen therein, and then the deionized water is redistilled. Then under the protection of nitrogen, weigh the stoichiometric ratio of sodium azide (NaN 3 ) and carbon tetrabromide (CBr 4 , 1.06 mol / liter) into the autoclave, add 5ml of deionized water after oxygen removal treatment and seal. Apply a pressure of 164.0 MPa on the autoclave, then control the temperature of the autoclave to rise to 300 °C at a rate of 0.93 °C / min, react at constant temperature and pressure for 6 hours and then naturally cool to room temperature.
[0043] After the reaction, filter the deionized water clean, then wash the product successively with acetone and dilute hydrochloric acid to remove organic by-products and other impurities therein, and then repeatedly wash the product with deionized water until the filtrate is neutral. Slowly heat the product in vacuum to 60°C for drying, and carbon nitri...
Embodiment 2
[0044] Embodiment 2: As described in Embodiment 1, the difference is that the protective gas is argon, the carbon source is carbon tetrachloride (content 0.005 mol / liter), the nitrogen source is potassium azide, and the reaction temperature is 240 ° C. The pressure is 20 MPa, the heating rate is 1°C / min, and the reaction time is 12 hours.
Embodiment 3
[0045] Embodiment 3: As described in Embodiment 1, the difference is that the protective gas is neon, the carbon source is bromoform (content 0.05 mol / liter), the nitrogen source is barium azide, and the reaction temperature is 350 ℃, and the pressure is 60MPa, the heating rate is 1.5°C / min, and the reaction time is 15 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com