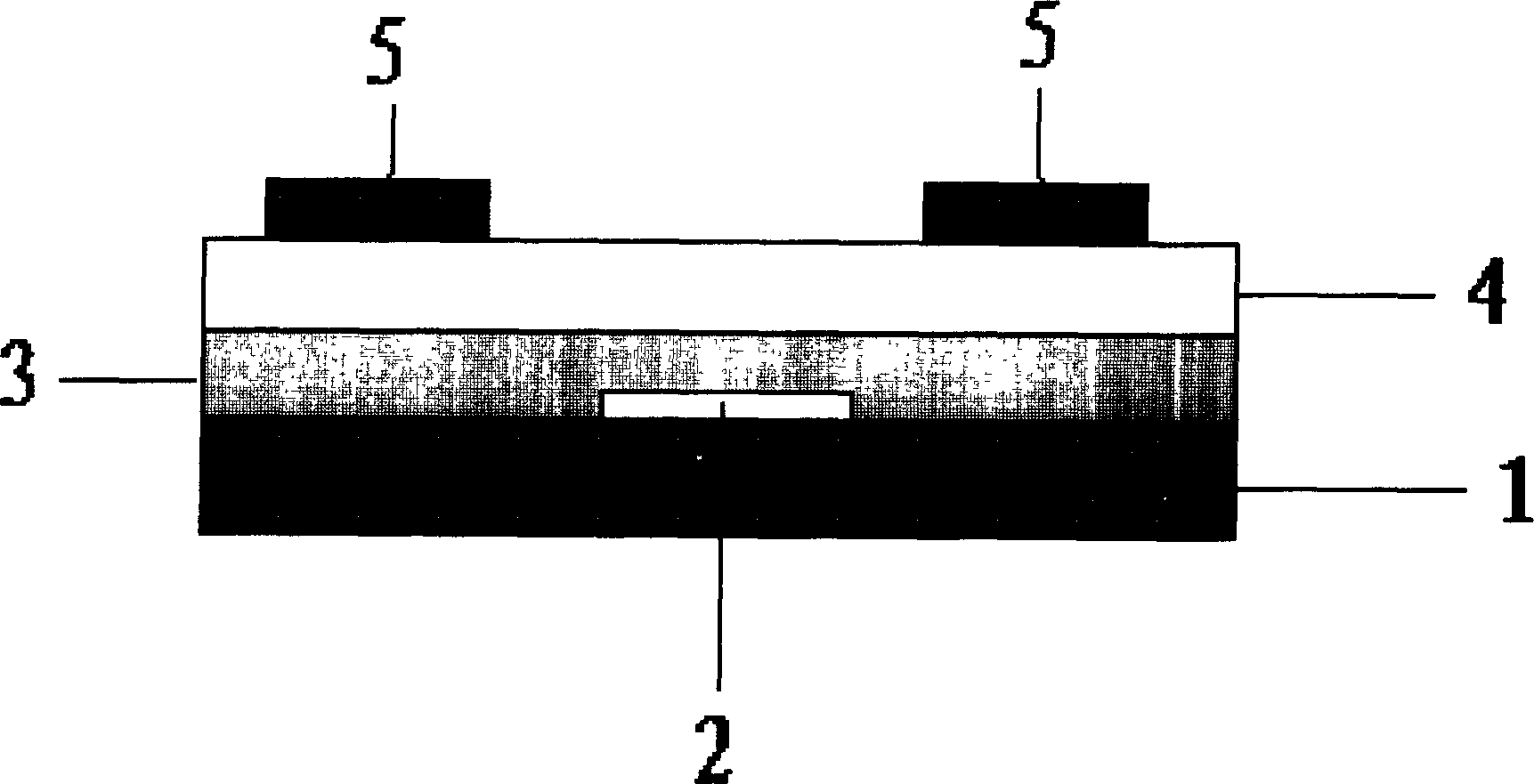
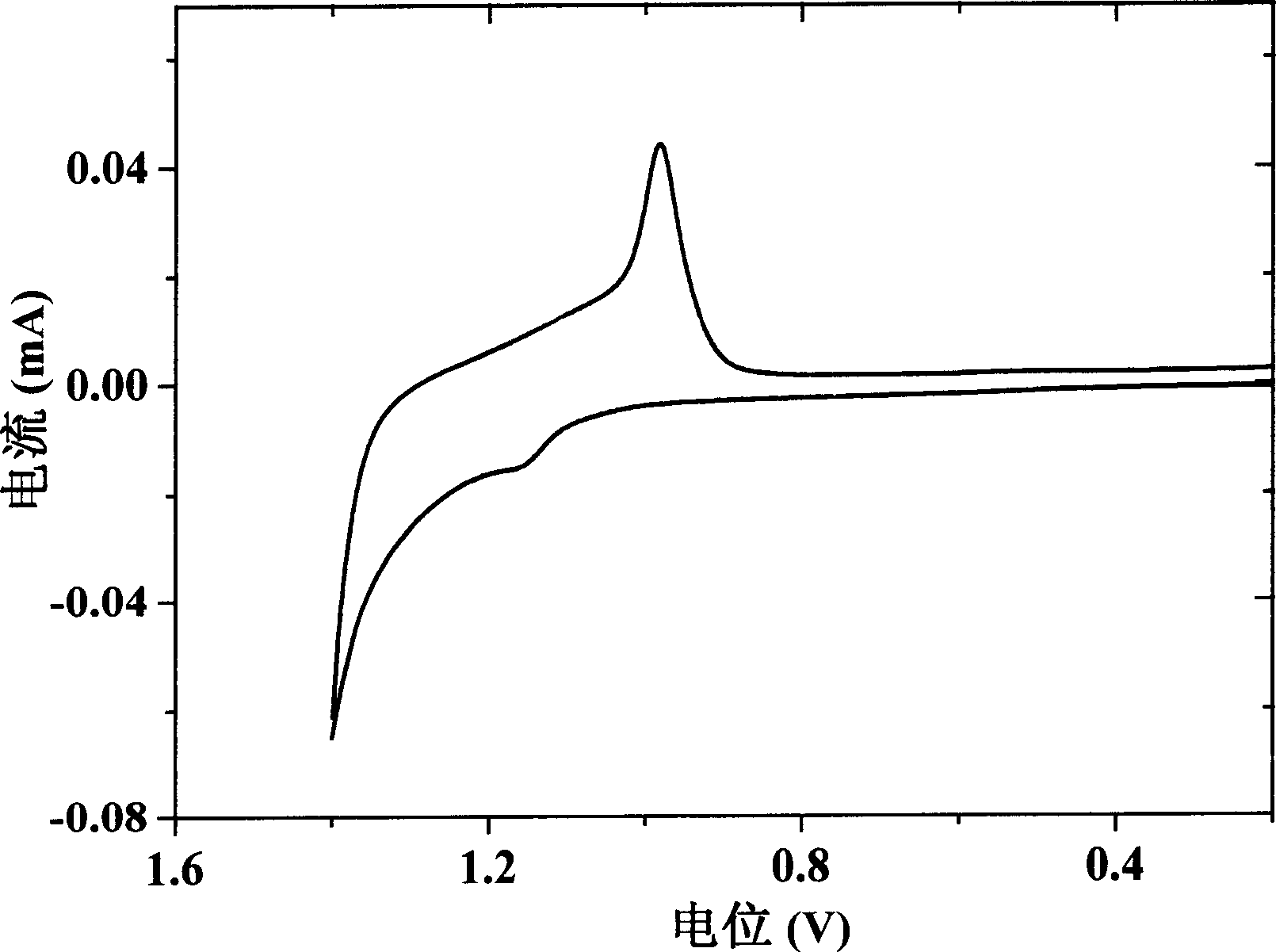
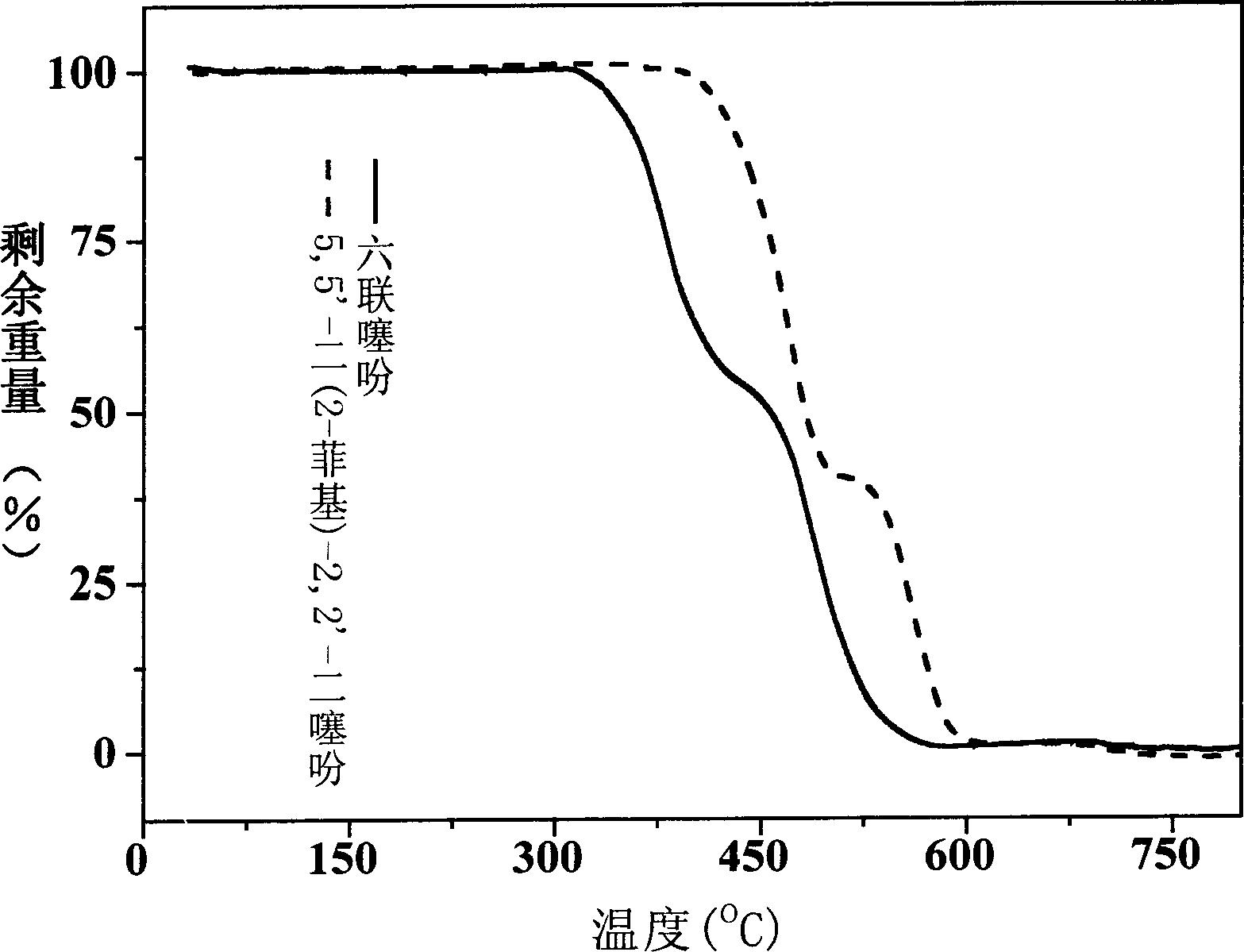
Phenanthrene/thiophene hybridized high-mobility organic semiconductor and application thereof
An organic semiconductor, high-mobility technology, applied in semiconductor devices, semiconductor/solid-state device manufacturing, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: the synthesis of 2-(2-phenanthrenyl)thiophene
[0044] Under the protection of argon, the 250ml round bottom flask connected to the double-row tube was baked three times with a gas lamp, and 7.69g (25.3mmol) of 2-iodophenanthrene, 8.58g (23.0mmol) of 2-(tributyltin base ) Thiophene, 500mg-1g of Pd (PPh 3 ) 4 , 80-150ml of DMF, the reaction mixture was stirred at 70-90°C for 5-10 hours, cooled to room temperature, poured into 500ml of water, extracted three times with 100ml of dichloromethane, combined organic phases, and separated with 100ml of saturated saline Wash three times, dry over anhydrous magnesium sulfate, remove the solvent by rotary evaporation, and separate and purify the obtained crude product by column chromatography, using petroleum ether as eluent to obtain 2-(2-phenanthrenyl)thiophene with a yield of 85%.
Embodiment 2
[0045] Embodiment 2: the synthesis of 2-bromo-5-(2-phenanthrenyl)thiophene
[0046] Under the protection of argon and avoiding light, add 4.79g (18.4mmol) of 2-(2-phenanthrenyl)thiophene to a 250ml three-necked flask, add 100-200ml of DMF that has been dried with calcium hydride, and place in an ice bath Within 0.5-1 hour, add 3.44g (19.3mmol) bromosuccinimide to the three-necked flask in portions, stir and react at this temperature for 1-2 hours, remove the ice bath, and continue stirring at room temperature for 2-2 hours. After 4 hours, add 500ml of water to end the reaction, continuously use 200ml of ether, wash three times with 100ml of saturated sodium thiosulfate solution respectively, dry the organic phase with anhydrous magnesium sulfate, and remove the solvent by rotary evaporation. The mixed solution of ether and dichloromethane was recrystallized to obtain 2-bromo-5-(2-phenanthrenyl)thiophene with a yield of 80%.
Embodiment 3
[0047] Example 3: Synthesis of 5,5'-bis(2-phenanthrenyl)-2,2'-dithiophene
[0048] Under the protection of argon, the 250ml round-bottomed flask connected to the double-row tube was baked three times with a gas lamp, and 4.79g (15.7mmol) of 2-iodophenanthrene, 5.32g (7.15mmol) of 5,5'-bis (Tributyltin base)-2,2'-dithiophene, 110-420mg of Pd(PPh 3 ) 4 , 60-200ml DMF, the reaction mixture was stirred at 70-100°C for 12-48 hours, cooled to room temperature, filtered to collect the precipitate, washed continuously with water, ethanol, dichloromethane, acetone, vacuum-dried, and the crude product was subjected to two Purified by sublimation in vacuum to obtain 5,5'-bis(2-phenanthrenyl)-2,2'-dithiophene with a yield of 50%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com