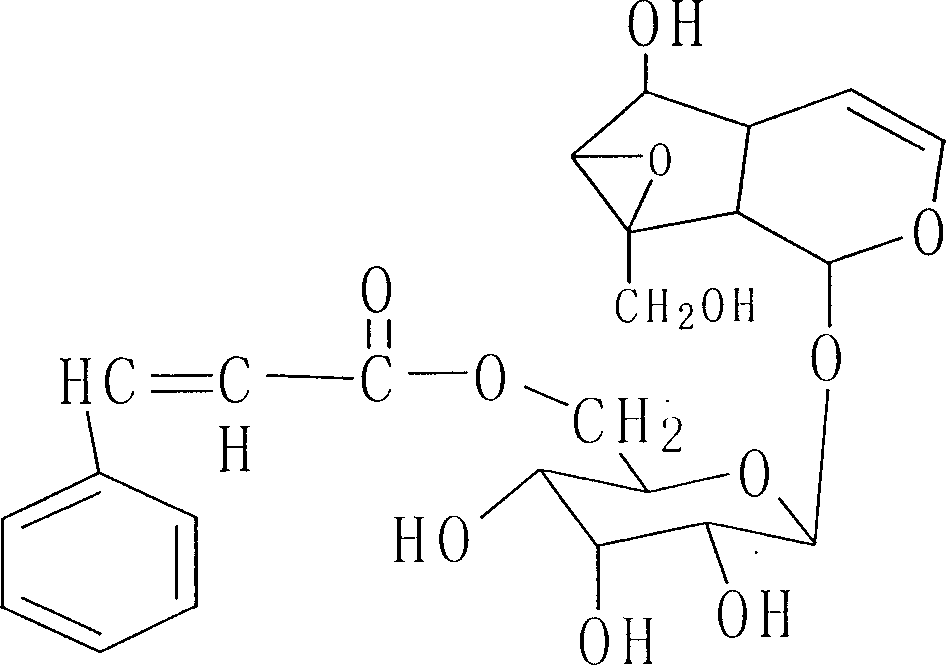
Method for determining kutkoside I content in biological sample
A technology for biological samples and picroside, which is applied in the preparation of test samples, measuring devices, instruments, etc., can solve the problems of complex pretreatment methods, poor reproducibility, low sensitivity, etc., and achieve shortened measurement time and high recovery rate. , the effect of good sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
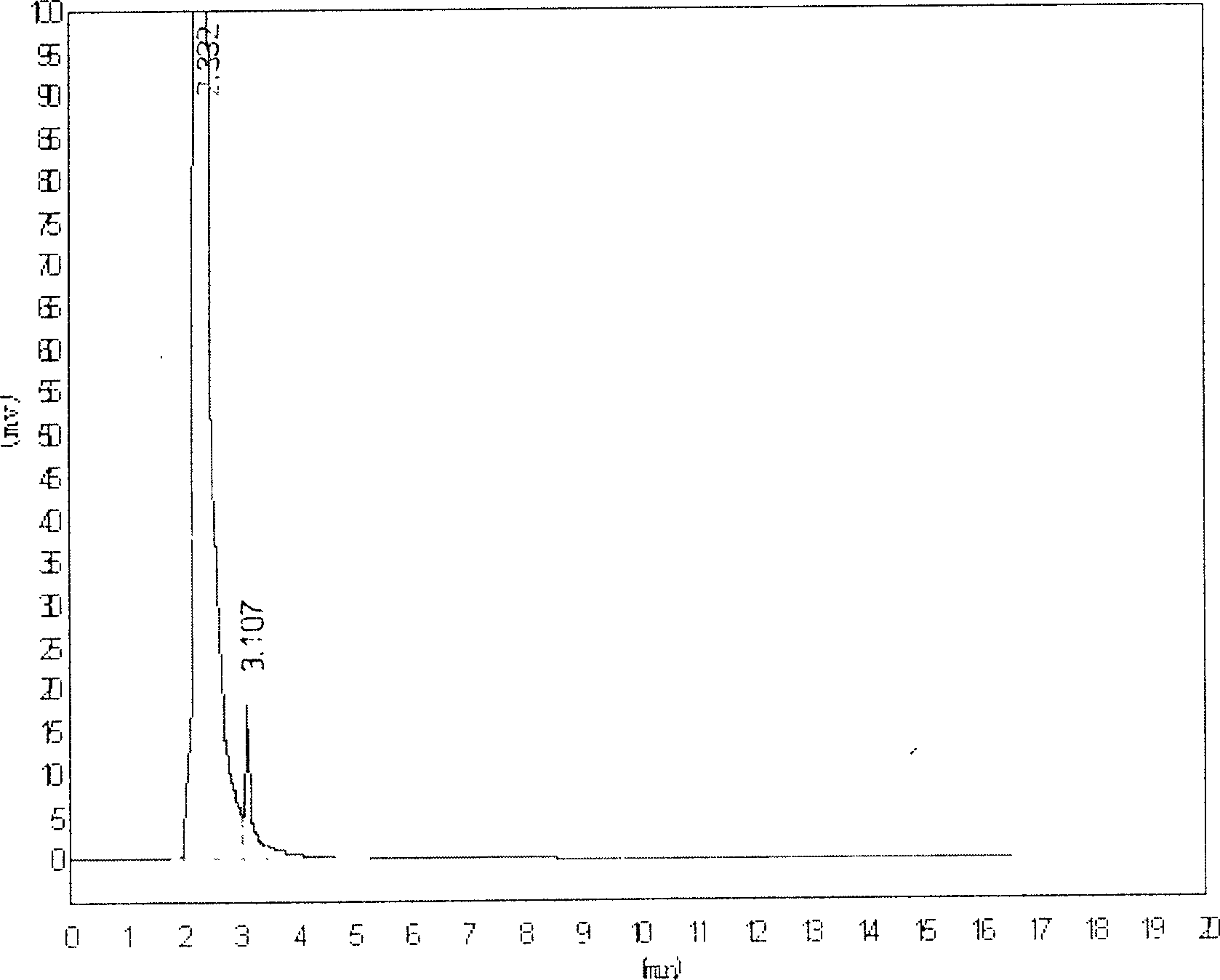
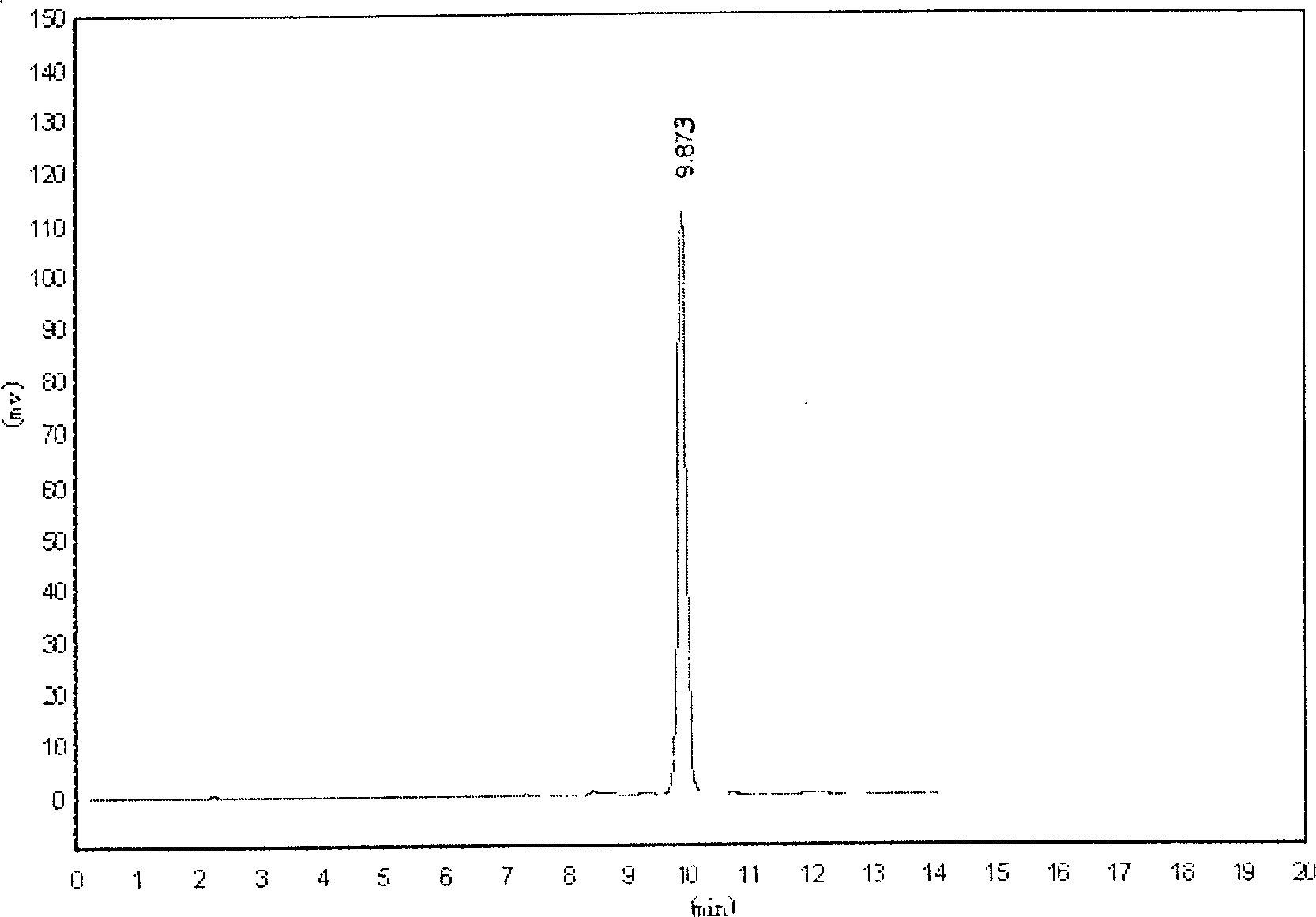
[0051] Example 1 The determination of the blood concentration of berberine-I freeze-dried powder for injection in rats
[0052] 1. Instruments and materials
[0053] 1.1 Instrument Shimadzu SPD-10AT VP Double-pump liquid chromatography; guard column: Shim-packGVP-ODS (10mm×4.6mm, 5μm) (Shimadzu Corporation of Japan); N2000 chromatographic workstation
[0054]1.2 Materials Piroside I reference substance (provided by Peking University Shijia Research Center, batch number: 010114); internal standard reference substance: paeoniflorin (National Institute for the Control of Pharmaceutical and Biological Products, batch number: 0736-200219); pyroside I freeze-dried powder Injection (provided by Peking University Shijia Research Center, batch number: 020912 specification 60mg / branch, purity: 96.63%); acetonitrile (chromatographically pure, DIKMA company); methanol (chromatographically pure); ascorbic acid, carbon tetrachloride (all prepared by Beijing Chemical Reagent Provided by th...
Embodiment 2
[0078] Example 2 Pirucetin-I freeze-dried powder injection is measured in blood concentration in Beagle dogs
[0079] Experimental animals were non-rodent Beagle dogs. Follow the same steps as described in Example 1.
[0080] The results showed that the intra-day and inter-day precision of the method was 5.23% and 6.19%, respectively, and the recovery rate was 91.73%-95.87% in the determination of berberine-I in Beagle dog plasma. After the intravenous injection of 2.5mg / kg of pipericolin-I in dogs, the 2min average blood concentration of berberine-I was 17.05μg / mL.
Embodiment 3
[0081] The concentration determination of embodiment 3 berberine-I freeze-dried powder injection in rat tissue
[0082] After injecting berberine-I 7mg / kg into the tail vein of rats, take heart, liver, spleen, lung, kidney, brain, stomach, intestine, uterus, testis, fat, muscle and other tissues and organs, and prepare tissue homogenate with normal saline , Determination after protein precipitation with acetonitrile. The chromatographic conditions are the same as in Example 1. The results showed that: berberine-I could be detected in all tissues, and the average drug concentration in kidney tissue was the highest at 5 minutes after administration, which was 66.61 μg / mL; the concentration in brain tissue was the lowest, at 0.65 μg / mL.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com