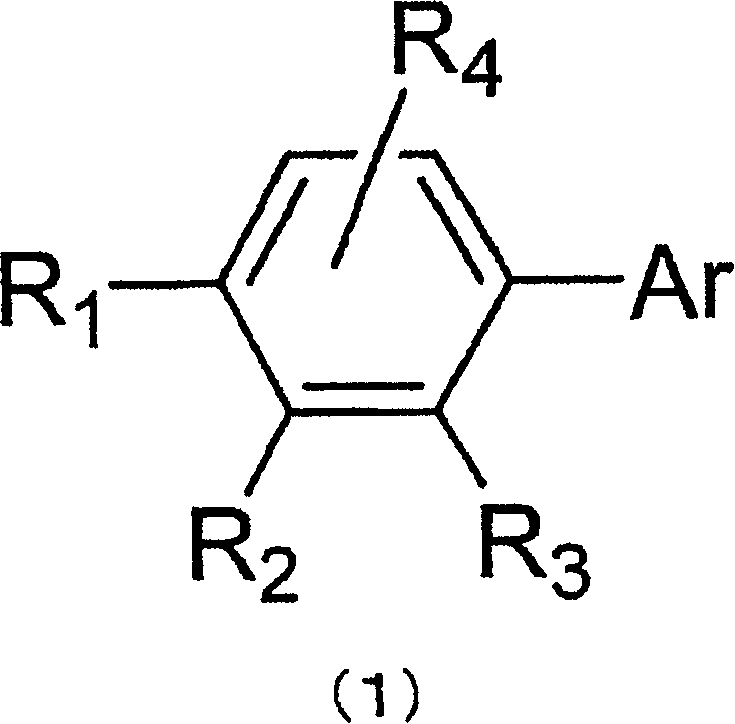
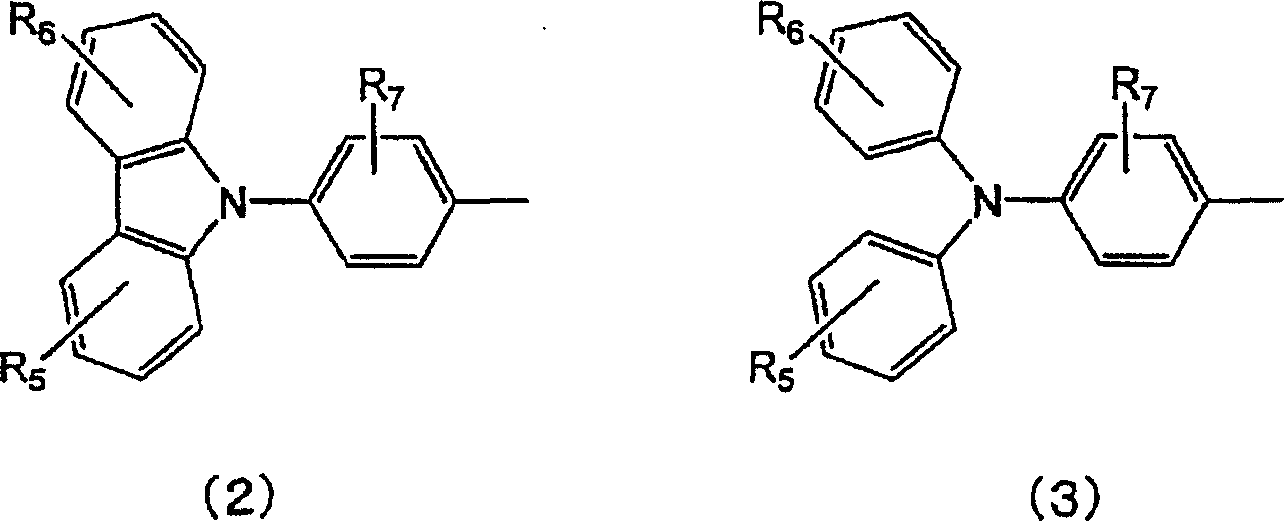
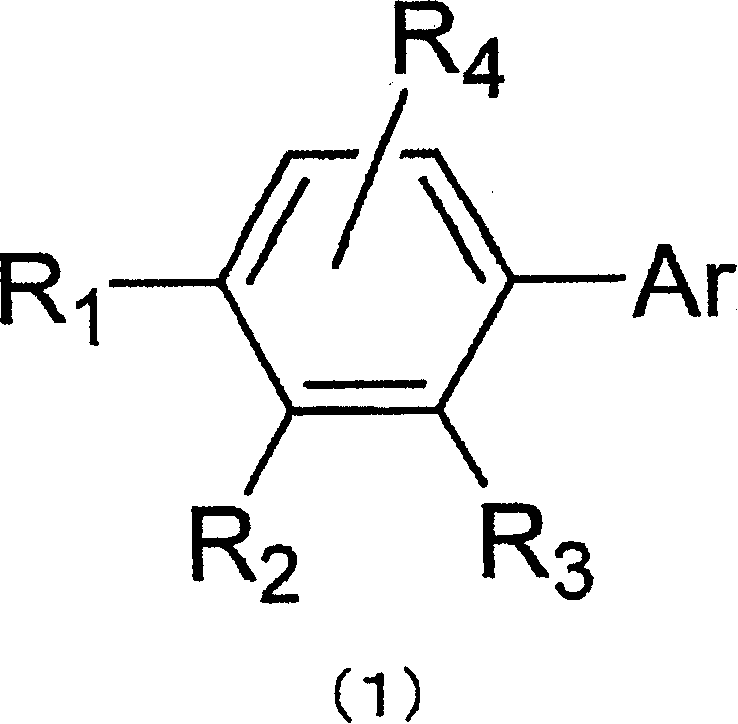
Material for organic electroluminescent element and organic electroluminescent element employing the same
An electroluminescent element and electroluminescent technology, applied in electroluminescent light sources, luminescent materials, electrical components, etc., can solve the problems of increased linearity, decreased luminous efficiency, and difficult to avoid crystallization, and achieve excellent heat resistance, The effect of long life and high luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0104] Next, the present invention will be described in further detail using examples, but the present invention is not limited to these examples.
[0105] Intermediate Synthesis Example 1 (4-(carbazol-9-yl)phenylboronic acid: (Intermediate A))
[0106] 254 g of 4-bromoiodobenzene (manufactured by Tokyo Kasei Co., Ltd.), 100 g of carbazole (manufactured by Tokyo Kasei Co., Ltd.), 1 g of copper iodide (manufactured by Wako Pure Chemical Industries, Ltd.), and 267 g of potassium phosphate (manufactured by Wako Pure Chemical Industries, Ltd.) were suspended in 1. To 500 ml of 4-dioxane (manufactured by Wako Pure Chemical Industries, Ltd.), 7 ml of trans-1,2-cyclohexanediamine was added, and the mixture was heated to reflux for 14 hours under an argon atmosphere.
[0107] The reaction solution was cooled to room temperature, dichloromethane and water were added to separate the two layers, and then the organic layer was washed with 5% hydrochloric acid and water, and dried with anhydro...
Synthetic example 3
[0116] Intermediate Synthesis Example 3 (Synthesis of 3,5-diphenylphenylboronic acid (Intermediate C))
[0117] To 150 g of 2,4,6-tribromoaniline (manufactured by Aldrich Corporation), 2.2 liters of water and 2.2 liters of concentrated hydrochloric acid were added, and the mixture was stirred at room temperature for 30 minutes. It was then cooled to 0°C, and a 120 ml solution of 60 g of sodium nitrite (manufactured by Wako Pure Chemical Industries, Ltd.) in water was added dropwise below 5°C. After the dripping, it was stirred at 5°C or lower for 1 hour, and a 150 ml water solution of potassium iodide 150 g (manufactured by Wako Pure Chemical Industries, Ltd.) was dropped at 5°C or lower. After stirring for 1 hour at 5°C or less, the precipitated crystals were filtered, washed with water, dried, and purified by silica gel column chromatography to obtain 165 g of 2,4,6-tribromoiodobenzene.
[0118] 160 g of this was dissolved in 1.6 liters of dehydrated THF (tetrahydrofuran) (manuf...
Synthetic example 1
[0120] Synthesis Example 1 (Compound (A-1)) Synthesis
[0121] Add 1.0 g of Intermediate B, 0.2 g of phenylboronic acid (manufactured by Tokyo Chemical Industry Co., Ltd.), and 0.1 g of tetrakis(triphenylphosphine)palladium (O) (manufactured by Tokyo Chemical Industry Co., Ltd.), and add 4 ml of 2M sodium carbonate aqueous solution and 7 ml of DME The mixed solution (manufactured by Kanto Chemical Co., Ltd.) was heated to reflux at 90°C for 10 hours.
[0122] After the completion of the reaction, it was placed at room temperature, filtered, and the crystals were washed with DME, water, and methanol successively and dried. It was further purified by column chromatography to obtain 0.72 g of white powder. Measure its FD-MS (field desorption mass spectrometry), the result is relative to C 48 H 32 N 2 =636, a peak of m / z=636 was obtained, so it was identified as the compound (A-1) (yield 73%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com