Device and method for the determination of protein domain boundaries
a technology of protein domain boundaries and devices, applied in the direction of peptide preparation methods, biochemistry apparatus and processes, biochemistry apparatus, etc., can solve the problems of difficult to recover remaining active enzymes for repeated use after the reaction, difficult to obtain suitable crystals, and high labor intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0067] General Experimental Protocol for Immobilizing Proteases on Microtiter Plates
[0068] In this example, four proteolytic enzymes are immobilized on a microtiter plate.
[0069] Reagents
[0070] The reagents were prepared as follows. A buffer solution of TBS, 50 mM Tris pH 8.0, 150 mM NaCl is prepared for use with the remaining solutions. A solution of TBS, 0.01% beta-octa-glucoside is prepared as a blocking buffer. The following proteases are prepared: Chymotrypsin, 0.5 mg / ml (in TBS); Trypsin, 0.5 mg / ml (in TBS); Papain, 0.5 mg / ml (in TBS); and Proteinase K, 0.5 mg / ml (in TBS). The protein to be digested is prepared at 65 .mu.g / ml (diluted in TBS).
[0071] Preparation of Immobilized Protease Plate
[0072] In this example of device preparation, Nunclon surface 96 well microtiter plates are used. The proteases are immobilized onto the plates by aliquotting 45 .mu.l TBS into each well. Six wells are used for each protease to generate a series for each protease, each series operating at dec...
example ii
[0080] Comparison of Immobilized Protease Digestion to Solution Protease Digestion
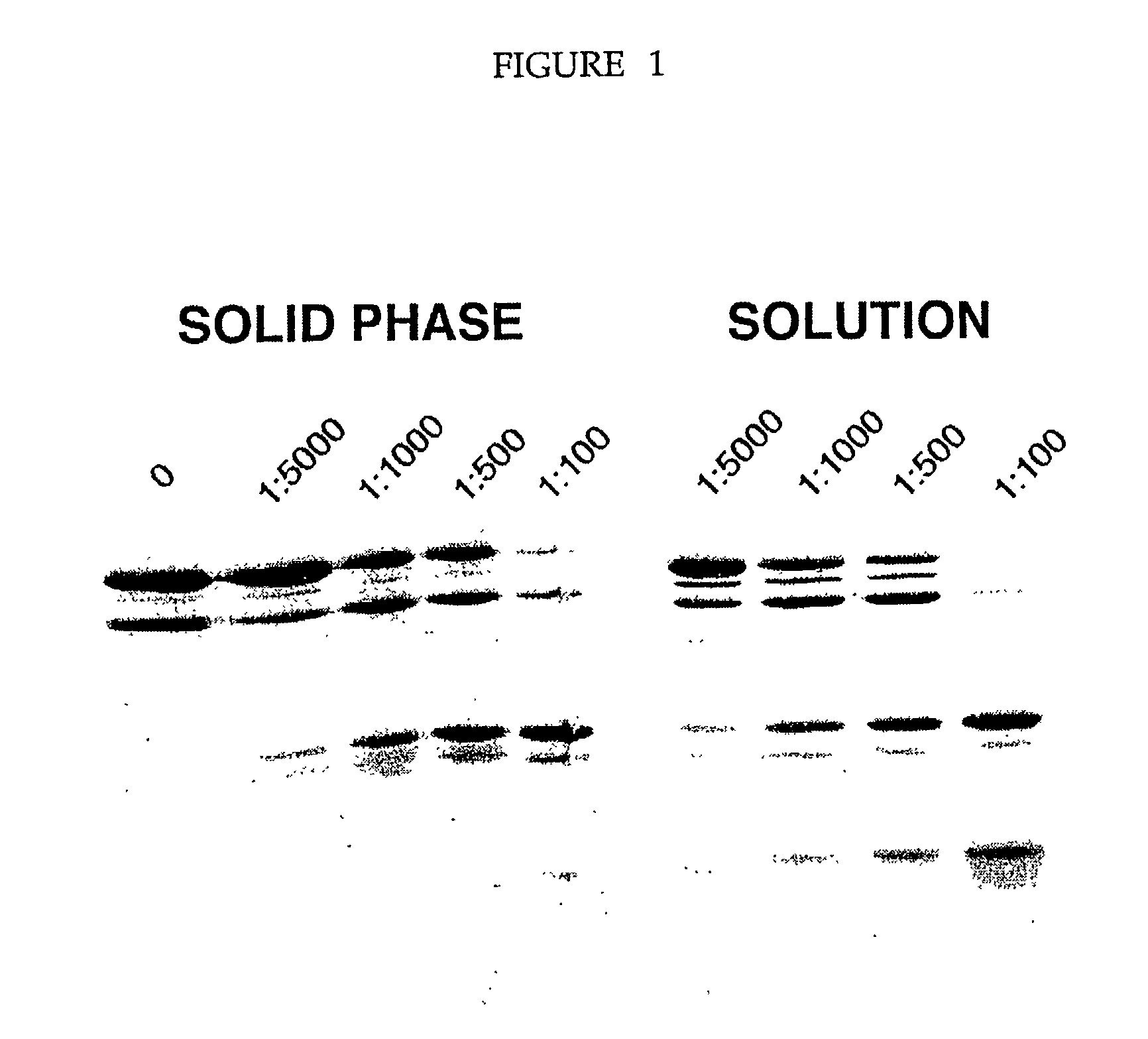
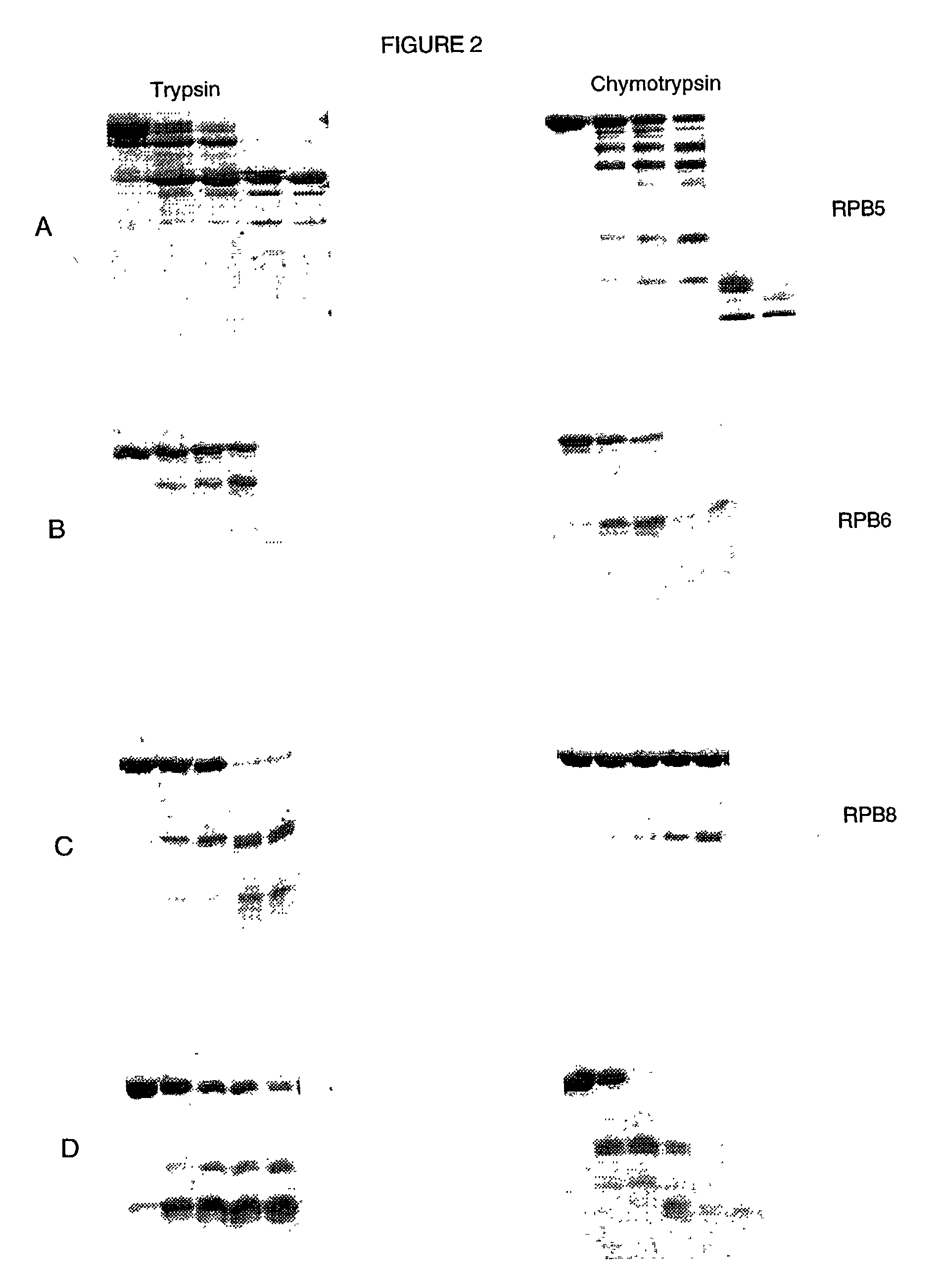
[0081] The protease plates were used to digest five different proteins whose proteolytic digestion pattern in solution was already known. The pattern of digestion for the immobilized proteases mimicked the solution digestion pattern. Thus, we have shown that the immobilized proteases plates could be used to identify stable domains of several different proteins. Shown in FIG. 1 is an example of a limited digestion of the transcription factor TFIIS from the yeast, Saccharomyces cerevisiae. The five lanes shown correspond to the concentration of proteases used (as discussed above in Example I). FIG. 2 illustrates results of digestion on immobilized protease plates of RNA polymerase B5 (see A); RNA polymerase B6 (B); RNA polymerase B8 (C); and D is a bacterial protein parB. Each of these four proteins were digestd by trypsin (panels on the left) and chymotrypsin (panels on the right). These digests compare...
example 111
[0082] Limited Proteolysis
[0083] Four different proteases, trypsin, chymotrypsin, papain and proteinase K (Sigma) were immobilized on plastic 96-well microtitre plates (Nuclon) in the following manner. The protease stocks were made 0.5 mg / ml in TBS (50 mM Tris pH 8, 150 mM NaCl). A serial dilution of each protease was prepared to final concentrations of 50 .mu.g / ml, 25 .mu.g / ml, 5 .mu.g / ml, 2.5 .mu.g / ml and 0.5 .mu.g / ml in TBS. 50 .mu.l of each dilution was applied to different wells in a row of the microtitre plate. The plate with the arrayed protease dilutions was then incubated overnight at 4.degree. C. in a sealed bag containing a wet paper towel.
[0084] The protease solution was then removed and the wells washed with 100 .mu.l of blocking buffer (TBS, 0.01% beta-octyl glucoside). The first wash was discarded and the non-specific binding sites on the microtitre wells were blocked with an additional 30 minute incubation at 4.degree. C. with an additional 100 .mu.l of blocking buff...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com