Methods and compositions for regulating cellular signaling
a technology of cellular signaling and composition, applied in the direction of instruments, transferases, genetic material ingredients, etc., can solve the problems of not previously addressed parts of the pkk function of the signaling pathway
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
[0342] PKK is Highly Related to RICK
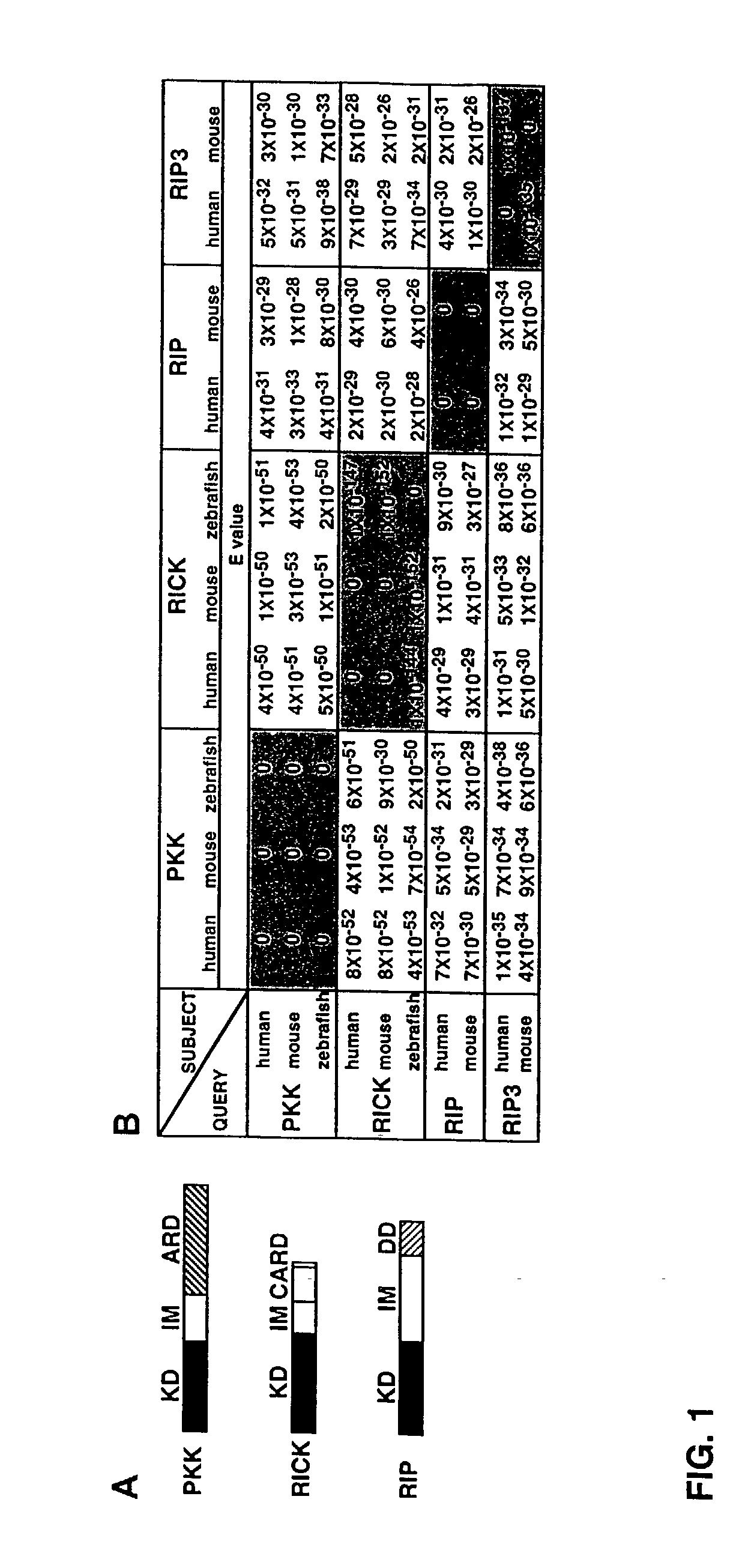
[0343] To identify novel RICK-like molecules, public protein and nucleotide databases were searched for homologous proteins using the entire RICK sequence (Inohara et al., J. Biol. Chem. 273:12296 [1998]). The search identified RIP (E values; 4.times.10.sup.-29 and 3.times.10.sup.-29 for human and mouse RIP, respectively) and its homologue RIP3 (E values; 1.times.10.sup.-31 and 5.times.10.sup.-30 for human and mouse RIP3, respectively) as molecules with significant homology to RICK (FIG. 1). In addition, the search identified PKK, a kinase of unknown function, as the most homologous protein to RICK in available databases (E=4.times.10.sup.-51 for mouse PKK and 4.times.10.sup.-10 for human PKK). The search also identified zebrafish orthologues of PKK and RICK. The domain structure of the fish PKK and RICK was identical to that of their mammalian orthologues (FIG. 1A). Zebrafish PKK was more homologous to human RICK (E=5.times.10.sup.-50) than human...
example 3
[0344] PKK Activates NF-.kappa.B and AP-1
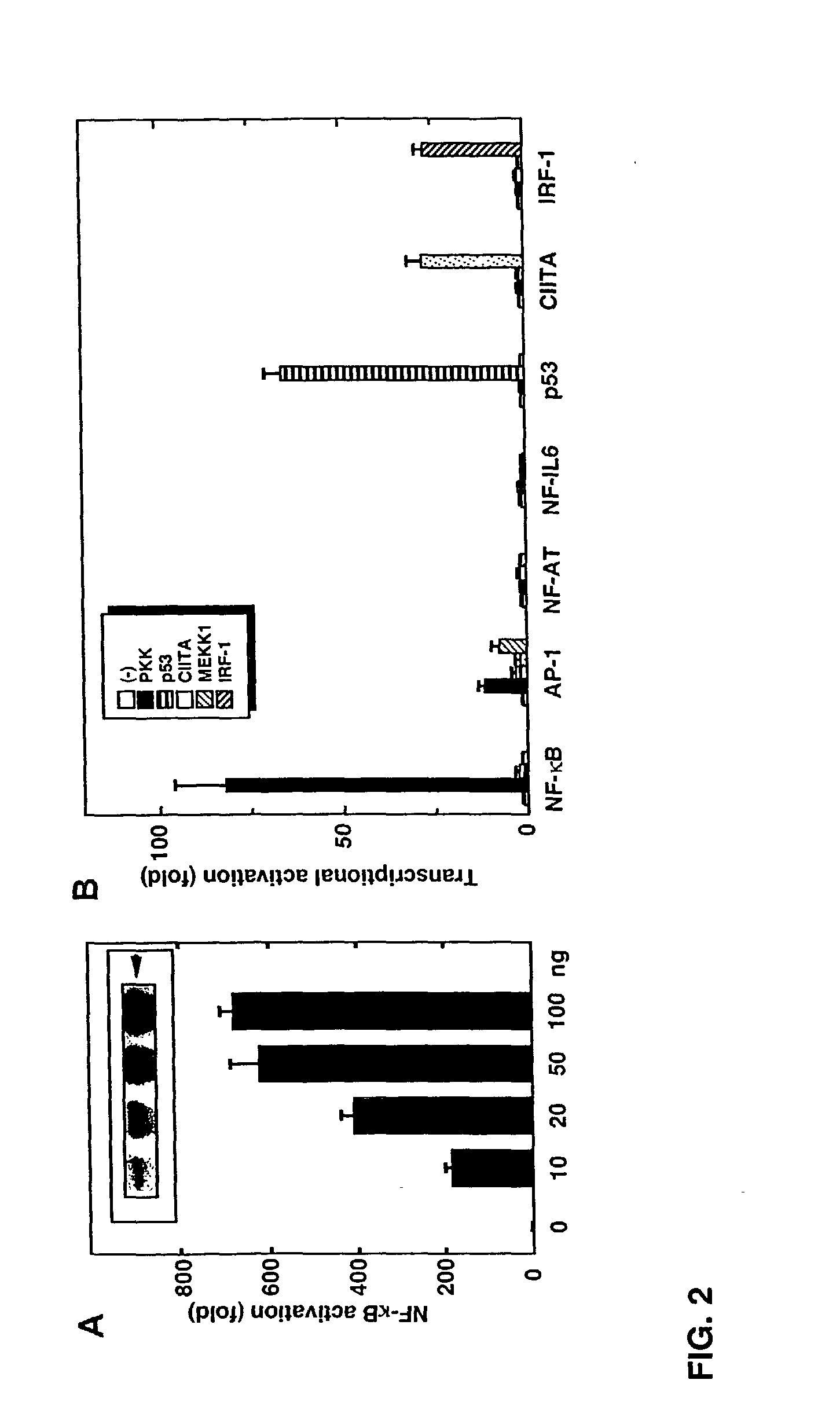
[0345] This example describes that the expression of PKK activates NF-.kappa.B. Transfection of the wild-type PKK cDNA into HEK293T cells induced activation of NF-.kappa.B in a dose-dependent manner, as measured with a reporter luciferase construct (FIG. 2A). The induction of NF-.kappa.B by PKK was specific in that transfection of the PKK cDNA did not induce transactivation of NF-AT, NF-IL6, p53, IRF-1 and class II MHC-dependent promoters (FIG. 2B). In control experiments, the transcriptional activity of the reporter constructs was stimulated by expression of proteins known to induce their activation (FIG. 2B). Expression of PKK induced significant activation of AP-1 (FIG. 2B) as did expression of MEKK1, a known activator of AP-1 (Xu et al., Proc. Natl. Acad. Sci. USA. 93:5291 [1996]).
example 4
[0346] The Kinase Domain of PKK is Essential for NF-.kappa.B Activation
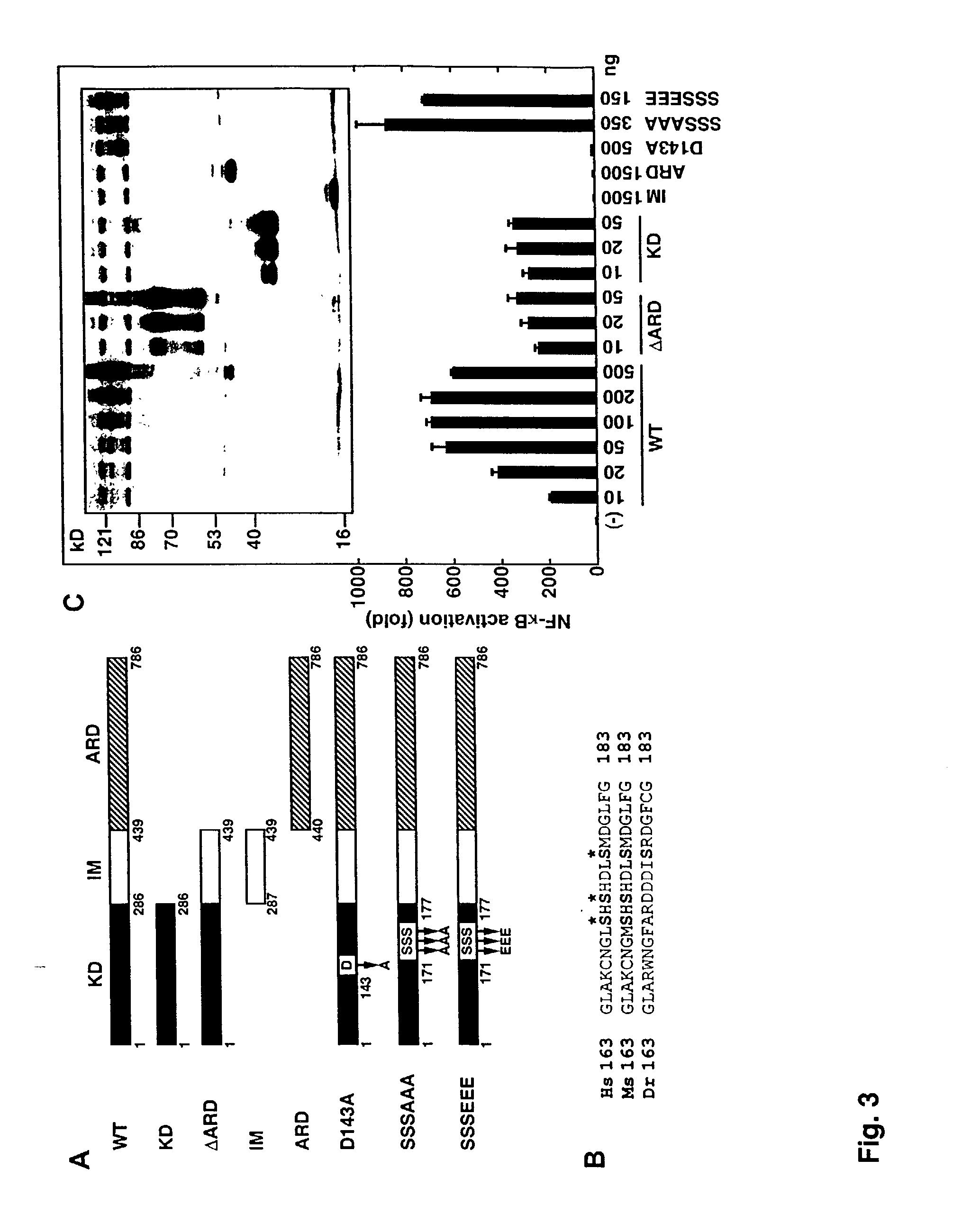
[0347] To identify the domains of PKK that are required for NF-.kappa.B activation, a series of deletion mutants carrying each domain alone or in combination were constructed (FIG. 3A). Expression of PKK mutants containing the kinase domain alone (SEQ ID NO: 27) or a combination with other domains such as the IM domain (SEQ ID NO: 28) resulted in NF-.kappa.B activation, while mutants containing the IM region (SEQ ID NO: 29) and / or ankyrin repeats-containing domain (ARD) alone (SEQ ID NO: 30) were inactive (FIG. 3C). Immunoblotting analysis showed that the lack of activity of the mutants could not be explained by-different expression levels of the mutant proteins (FIG. 3C, inset). Thus, the kinase domain of PKK is necessary and sufficient for NF-.kappa.B activation. The present invention is not limited to a particular mechanism. Indeed an understanding of the mechanism is not necessary to practice the present inve...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com