Negative electrode for nonaqueous secondary battery, process of producing the negative electrode, and nonaqueous secondary battery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1-1
(1) Preparation of Active Material Particles
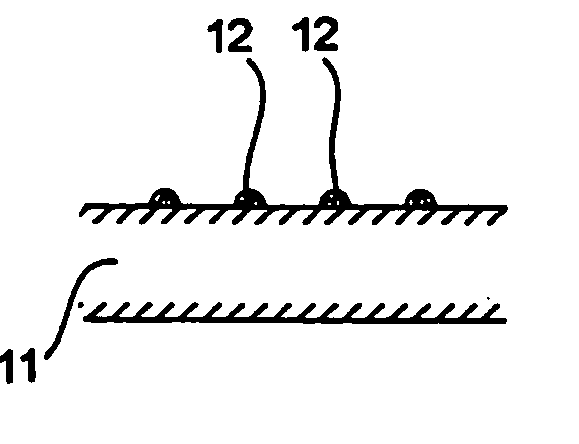
[0102] A molten metal at 1400° C. containing 90% of silicon and 10% of nickel was cast into a copper-made mold and quenched to obtain an ingot of a silicon-nickel alloy. The ingot was pulverized and sieved to obtain silicon-nickel alloy particles having particle sizes of 0.1 to 10 μm. The silicon-nickel alloy particles and nickel particles (particle size: 30 μm) were blended at a rate of 80%:20% and mixed and pulverized simultaneously in an attritor to obtain uniformly mixed particles of silicon-nickel particles and nickel. The mixed particles had the maximum particle size of 1 μm and a D50 value of 0.8 μm.
(2) Preparation of Slurry
[0103] A slurry having the following composition was prepared.
Mixed particles obtained in (1) above16%Acetylene black (particle size: 0.1 μm) 2%Binder (polyvinylidene fluoride) 2%Diluting solvent (N-methylpyrrolidone)80%
(3) Formation of Active Material Layer
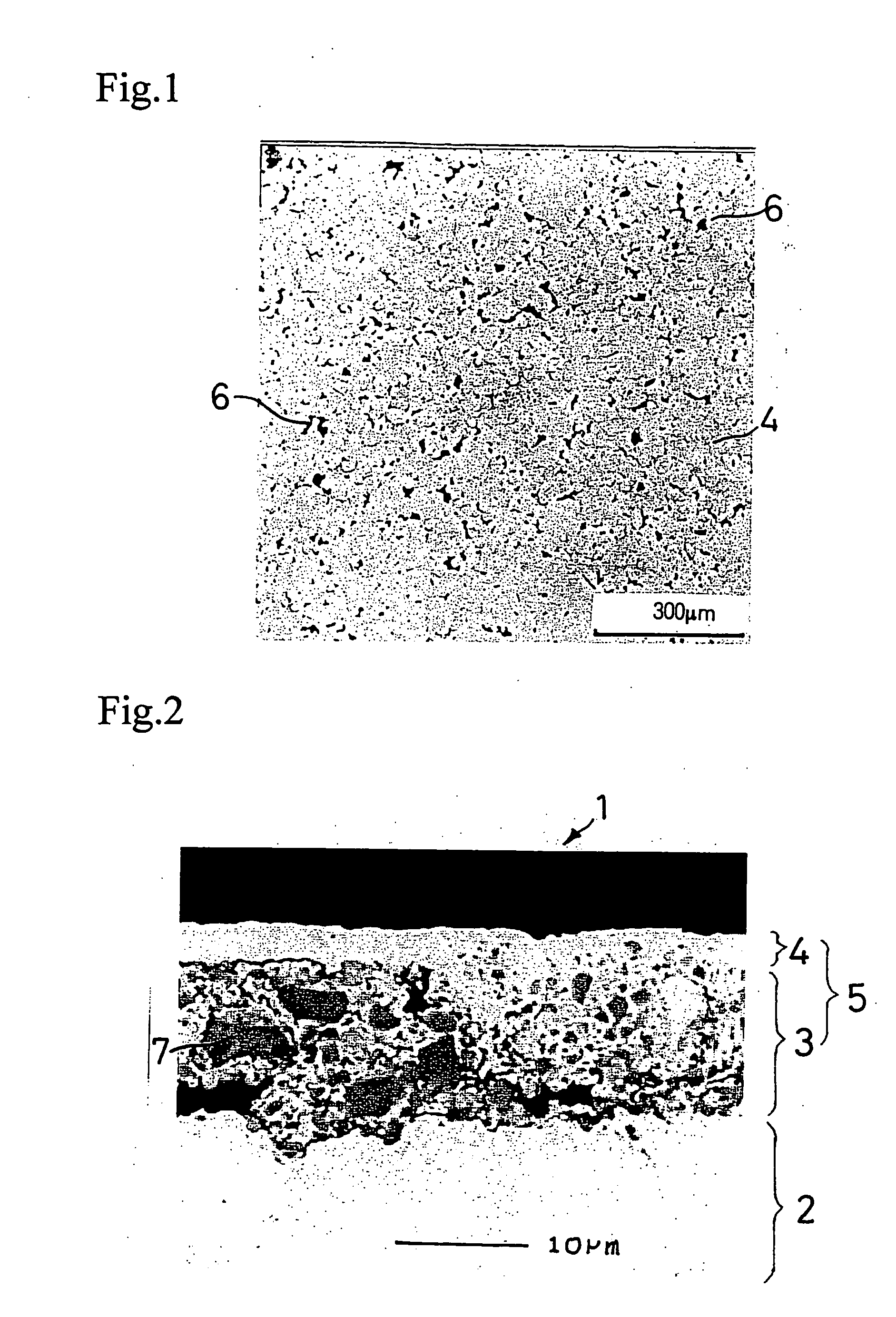
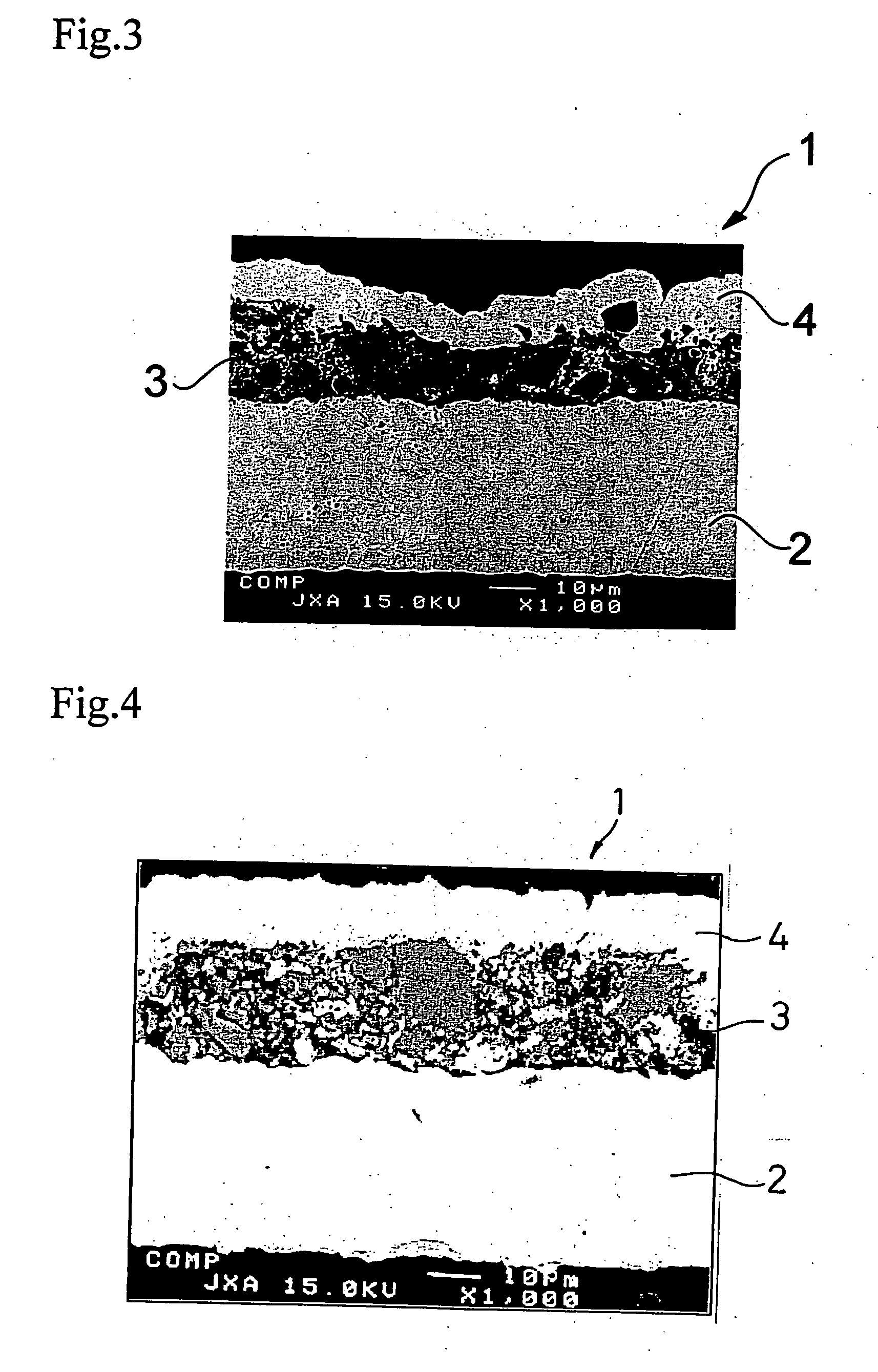
[0104] The above prepared slurry was applied to...
examples 1-2 to 1-4
[0107] A negative electrode was produced in the same manner as in Example 1-1, except for using the active material particles shown in Table 1-1 below. The same electron microscopic observation as in Example 1-1 revealed presence of micropores in the resulting negative electrode.
example 1-5
[0108] A 35 μm thick copper foil was plated with nickel to a deposit thickness of 2 μm to prepare a current collector. An active material layer and a surface coating layer were formed on the nickel layer in the same manner as in Example 1-1, except for using the active material particles shown in Table 1-1 in the active material layer. The same electron microscopic observation as in Example 1-1 revealed presence of micropores in the resulting negative electrode.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com