Process for preparation of alicyclic diepoxy compound, curable epoxy resin compositions, epoxy resin compositions for the encapsulation of electronic components, stabilizers for electrical insulating oils, and casting epoxy resin compositions for electrical insulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
Examples and Comparative Examples for Invention (1)
[0177] The following examples are used for illustrations of the present invention, and they do not restrict the scope at all.
example 1
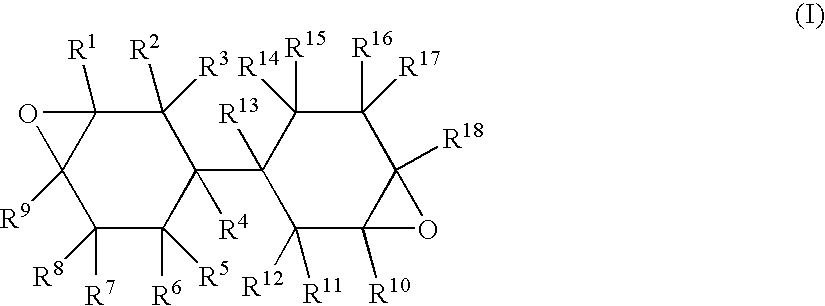
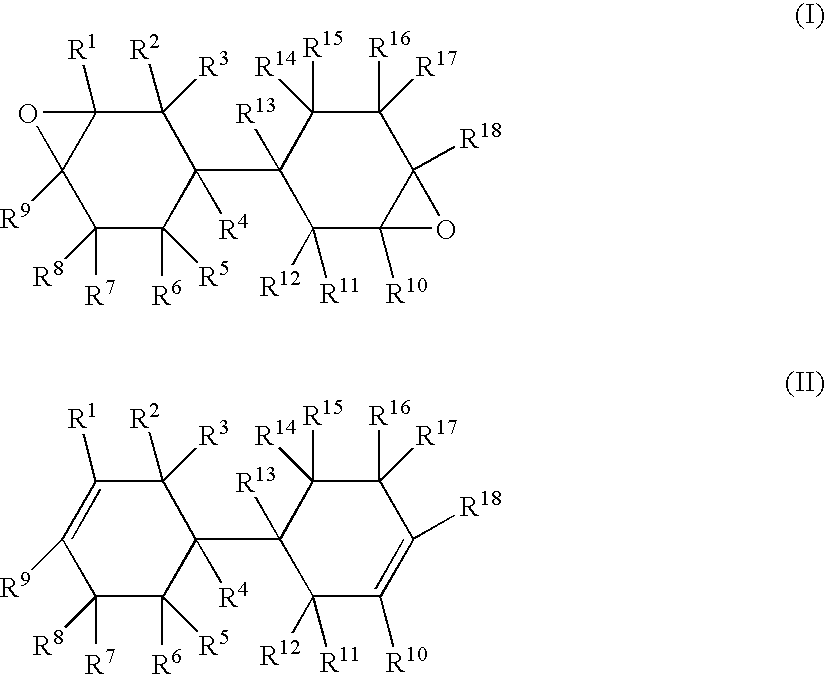
[0178] In a reactor, 406 g of bicyclohexyl-3,3′-diene, which is an alicyclic olefin compound represented by the above general formula (II), and 1,217 g of ethyl acetate were placed. While nitrogen was flown into a gas phase part and the temperature in the reaction system was controlled at 37.5° C., 457 g of an ethyl acetate solution (water content: 0.41% by weight) containing peracetic acid at 30% by weight was added dropwise for about 3 hours. After the completion of dropwise addition of the peracetic acid solution, the resulting mixture was aged at 40° C. for 1 hour to complete the reaction. The crude liquid at the completion of the reaction was then washed with water at 30° C., and treated at 70° C. / 20 mmHg to remove low-boiling-point compounds. Consequently, 415 g of an epoxy compound was obtained with an yield of 85%.
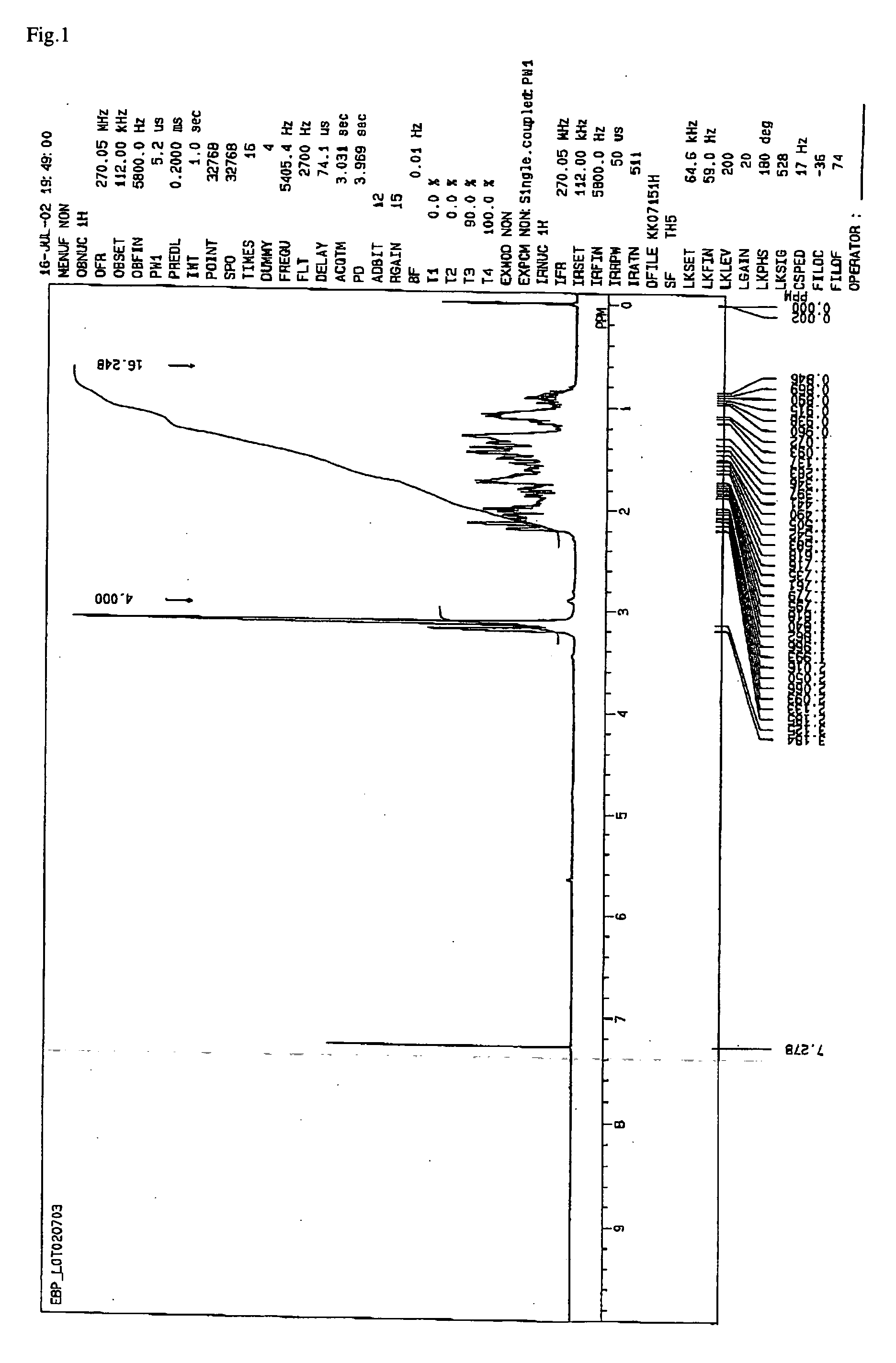
[0179] The oxirane oxygen content of the epoxy compound obtained was 14.7% by weight (theoretical value: 16.5% by weight).
[0180] In 1HNMR analysis, a peak at abo...
example 2
[0181] 243 g of bicyclohexyl-3,3′-diene, which is an alicyclic olefin compound represented by the above general formula (II), and 730 g of ethyl acetate were placed. While nitrogen was flown into a gas phase part and the temperature in the reaction system was controlled at 37.5° C., 274 g of an ethyl acetate solution (water content: 0.41% by weight) containing peracetic acid at 30% by weight was added dropwise for about 3 hours. After the completion of dropwise addition of peracetic acid solution, the resulting mixture was aged at 40° C. for 1 hour to complete the reaction. The crude liquid at the completion of the reaction was then washed with water at 30° C., and treated at 70° C. / 20 mmHg to remove low-boiling-point compounds. Consequently, 270 g of an epoxy compound was obtained with an yield of 93%.
[0182] The oxirane oxygen content of the epoxy compound obtained was 15.3% by weight.
[0183] In 1HNMR analysis, a peak at about δ4.5 to 5 ppm originating from the inner double bond d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com