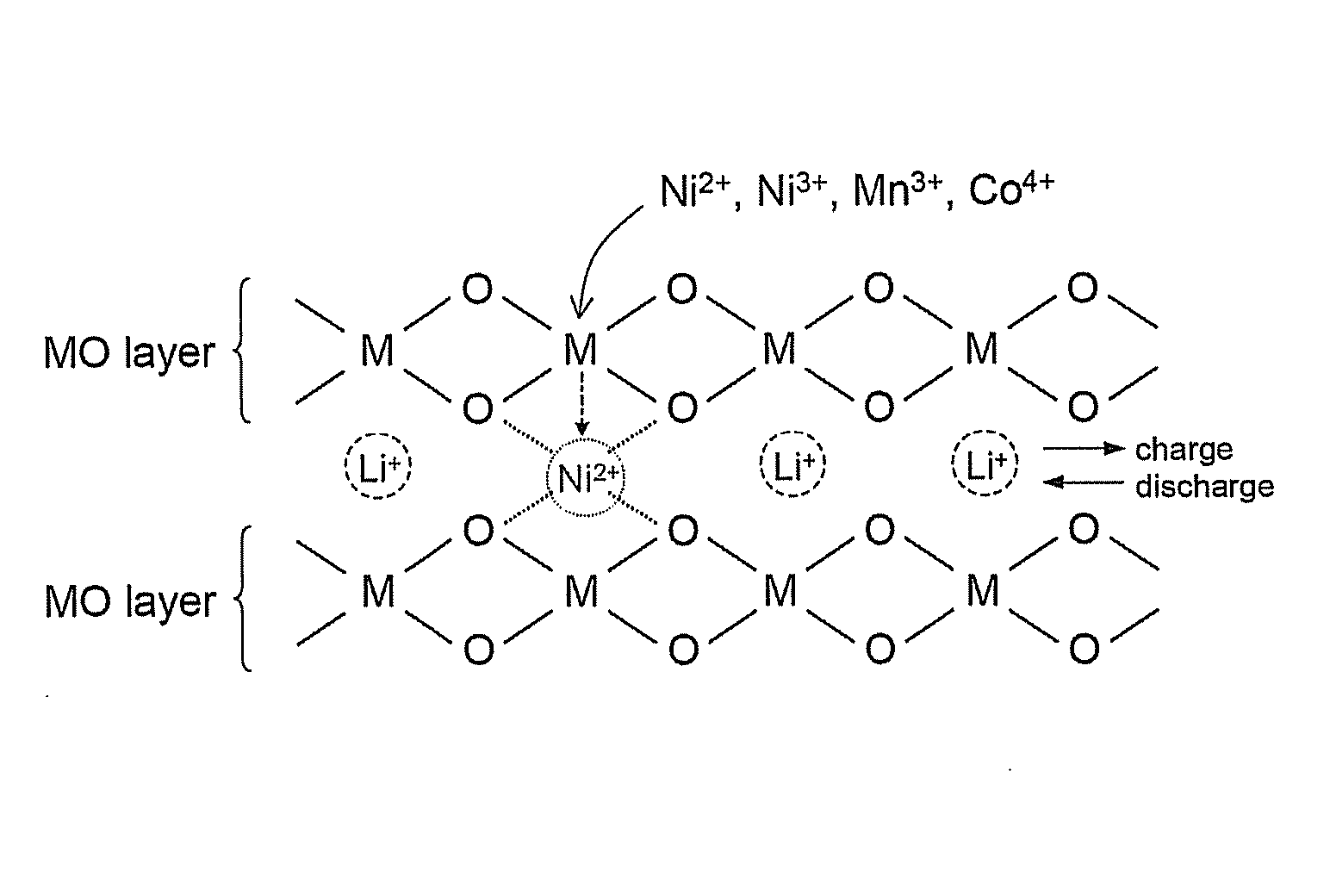
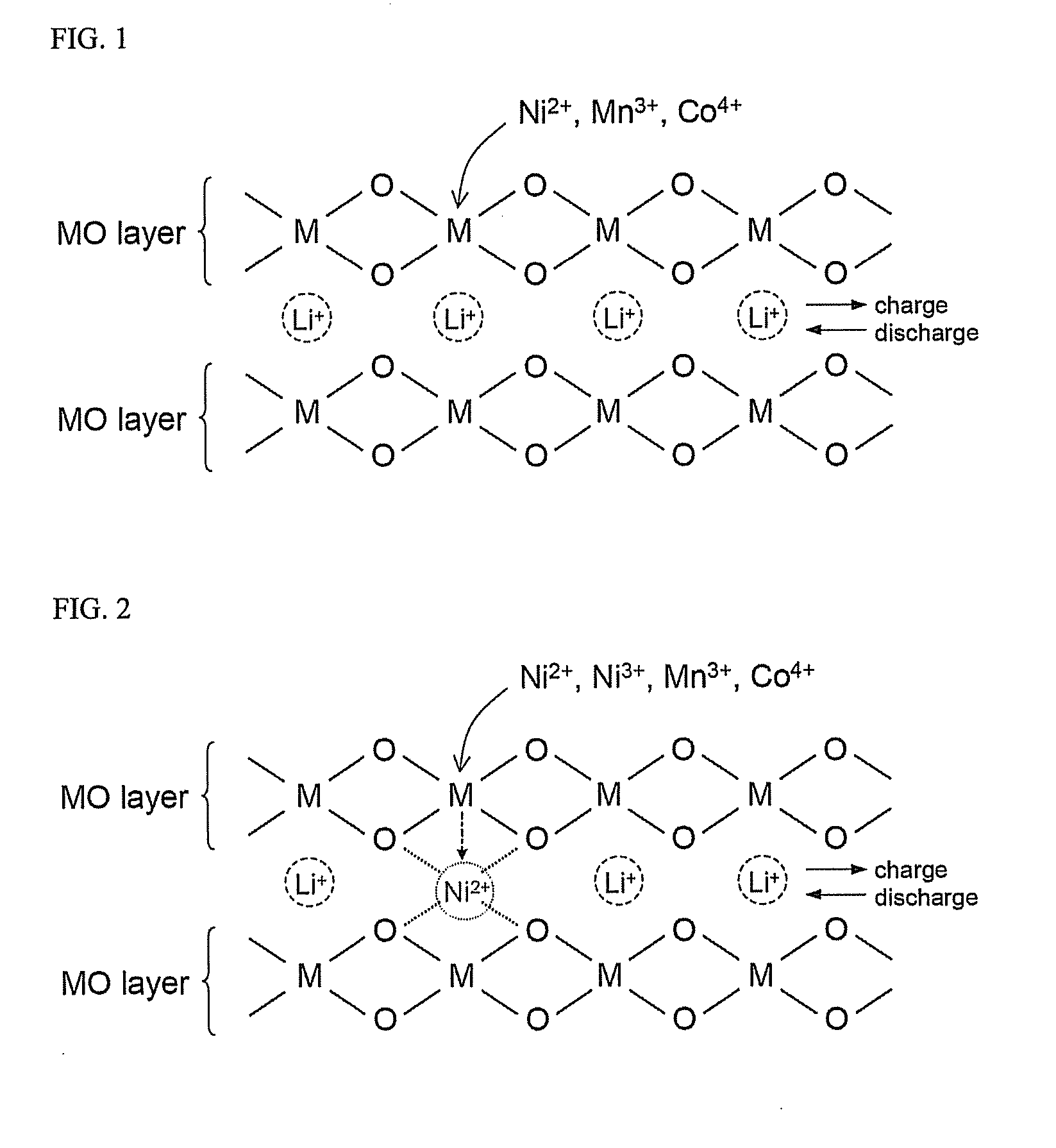
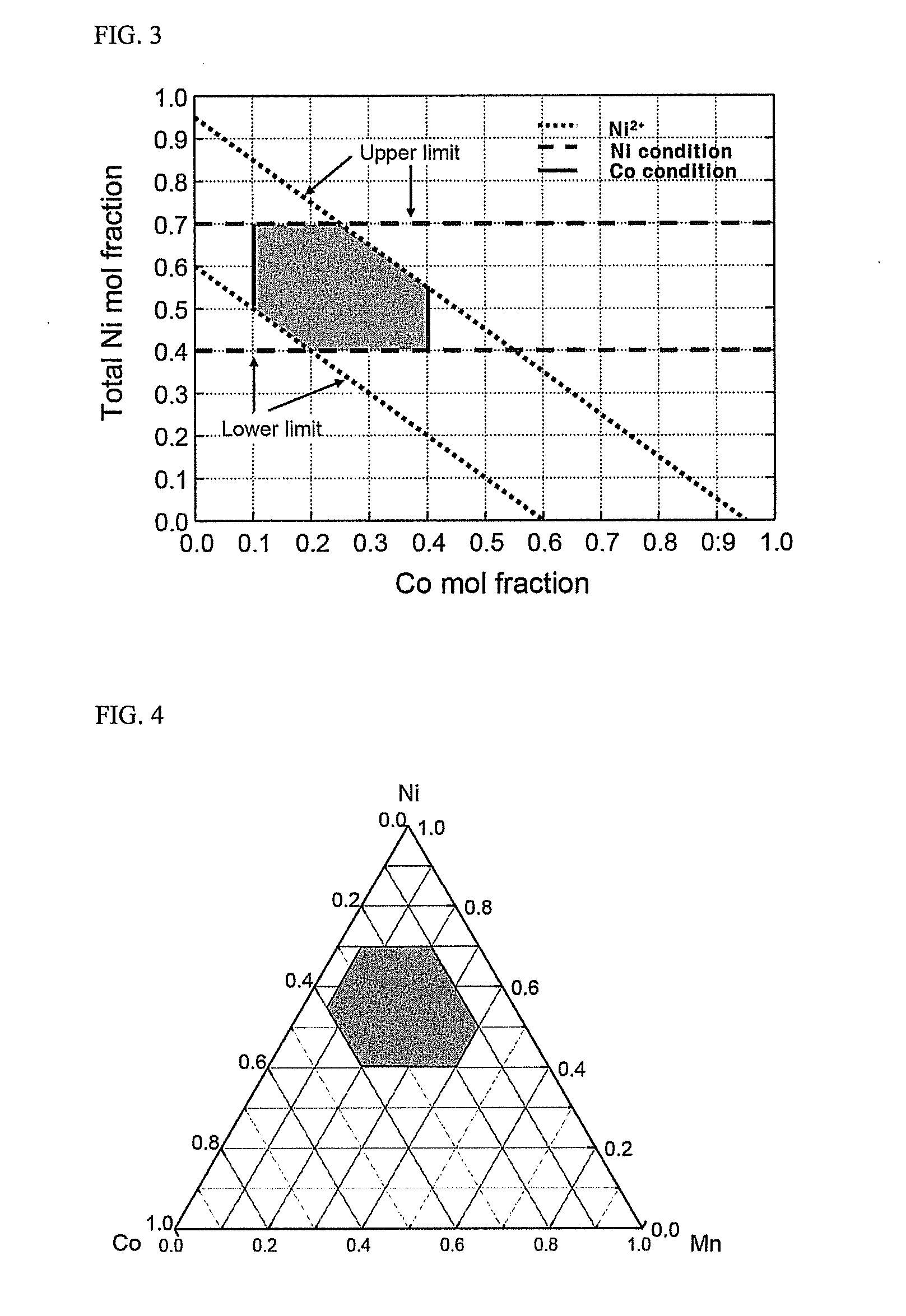
Material for lithium secondary battery of high performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0107] A mixed oxyhydroxide of Formula MOOH (M=Ni4 / 15(Mn1 / 2Ni1 / 2)8 / 15Co0.2) as a mixed transition metal precursor and Li2CO3 were mixed in a stoichiometric molar ratio (Li:M=1.02:1), and the mixture was sintered in air at temperatures of 850 (Ex. 1A), 900 (Ex. 1B), 950 (Ex. 1C), and 1,000° C. (Ex. 1D) for 10 hours, to prepare a lithium mixed transition metal oxide. Herein, secondary particles were maintained intact without being collapsed, and the crystal size increased with an increase in the sintering temperature.
[0108] X-ray analysis showed that all samples have a well-layered crystal structure. Further, a unit cell volume did not exhibit a significant change with an increase in the sintering temperature, thus representing that there was no significant oxygen-deficiency and no significant increase of cation mixing, in conjunction with essentially no occurrence of lithium evaporation.
[0109] The crystallographic data for the thus-prepared lithium mixed transition metal oxide are ...
example 2
[0116] The pH titration was carried out for a sample of the lithium mixed transition metal oxide in accordance with Example 2 prior to exposure to moisture, and samples stored in a wet chamber (90% RH) at 60° C. in air for 17 hours and 3 days, respectively. The results thus obtained are given in FIG. 9.
[0117] Upon comparing the lithium mixed transition metal oxide of Example 2 (see FIG. 9) with the sample of Comparative Example 3 (see FIG. 8), the sample of Comparative Example 3 (stored for 17 hours) exhibited consumption of about 20 mL of HCl, whereas the sample of Example 2 (stored for 17 hours) exhibited consumption of 10 mL of HCl, thus showing an about two-fold decrease in production of the water-soluble bases. Further, in 3-day-storage samples, the sample of Comparative Example 3 exhibited consumption of about 110 mL of HCl, whereas the sample of Example 2 exhibited consumption of 26 mL of HCl, which corresponds to an about five-fold decrease in production of the water-solubl...
example 3
[0119] Samples with different Li:M molar ratios were prepared from MOOH (M=Ni4 / 15(Mn1 / 2Ni1 / 2)8 / 15Co0.2). Li2CO3 was used as a lithium source. Specifically, 7 samples each of about 50 g with Li:M ratios ranging from 0.925 to 1.12 were prepared by a sintering process in air at a temperature of 910 to 920° C. Then, electrochemical properties were tested.
[0120] Table 3 below provides the obtained crystallographic data. The unit cell volume changes smoothly according to the Li:M ratio. FIG. 10 shows its crystallographic map. All samples are located on a straight line. According to the results of pH titration, the content of soluble base slightly increased with an increase of the Li:M ratio, but the total amount thereof was small. Accordingly, the soluble base probably originates from the surface basicity (i.e., is present by an ion exchange mechanism) but not from the dissolution of Li2CO3 impurities as observed in Comparative Example 1.
[0121] Therefore, this experiment clearly shows t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com