Compositions comprising high concentration of biologically active molecules and processes for preparing the same
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
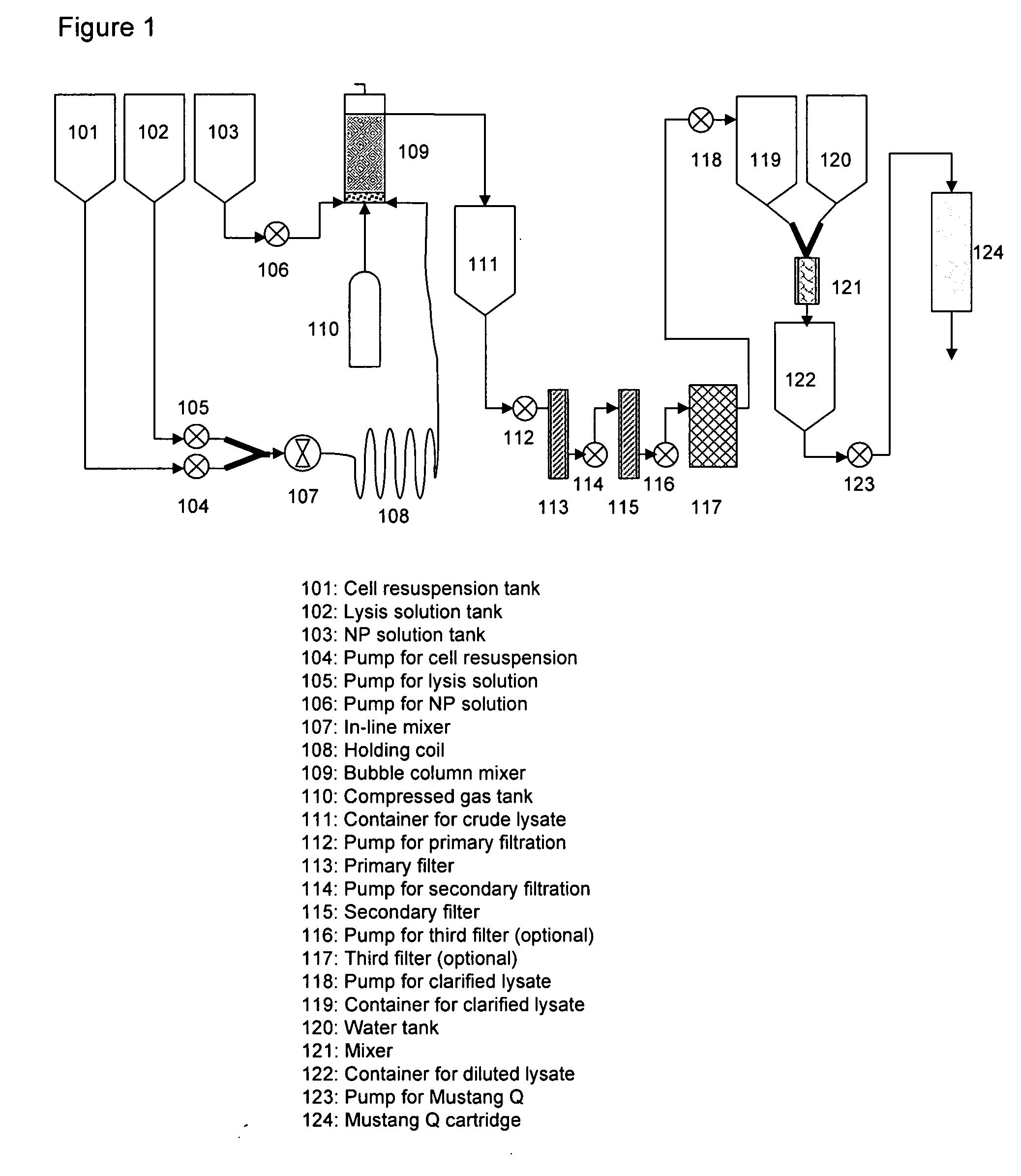
[0081]Escherichia coli (“E. coli”) cells containing a plasmid A were fermented to high density of an optical cell density (“OD600”) at 72 when harvested by centrifugation. Plasmid A has a size of 6549 bp. The plasmid typically replicates at a low copy number of ˜250 copies / cell. Approximately 3.1 kg wet cell weight (“WCW”) of cell paste was suspended in a resuspension buffer consisting of 25 mM Tris-hydrochloride (“Tris-HCl”, J. T. Baker, Phillipsburg, N.J.), 10 mM edetate disodium (“Na2EDTA”, Fisher Scientific, Fair Lawn, N.J.), pH 8, to a final volume of approximately 21.5 L. This cell suspension was pumped at 300 mL / min into one side of a “Y” connector. Simultaneously, lysis solution consisting of 0.2 N sodium hydroxide (“NaOH”, J. T. Baker, Phillipsburg, N.J.), 1% sodium dodecyl sulfate (“SDS”, J. T. Baker, Phillipsburg, N.J.) was pumped at 300 mL / min into the other side of the “Y” connector. The combined fluids exiting the “Y” connector were immediately passed through a Silvers...
example 2
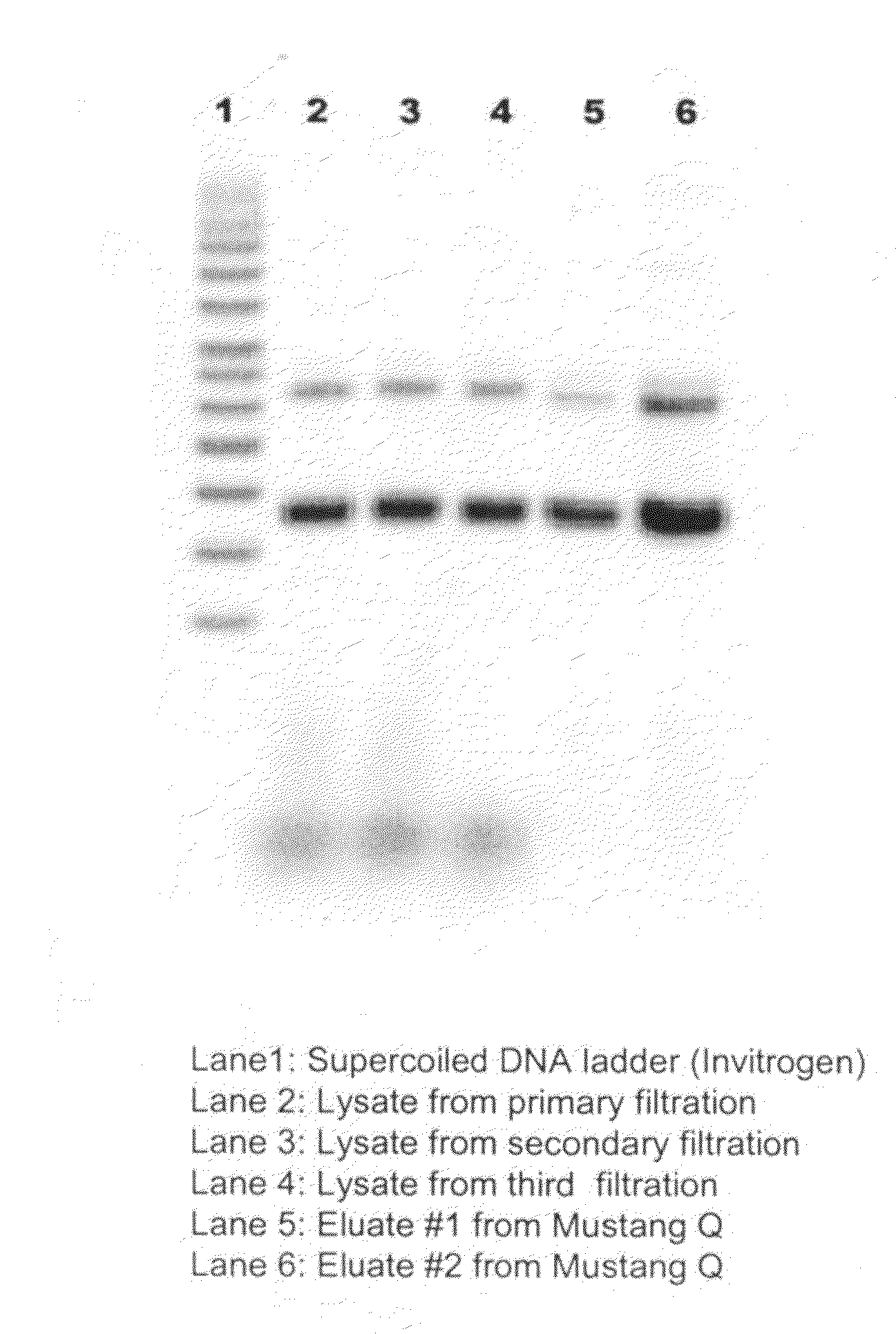
[0091]An amount of 3140 gram E. coli cells containing plasmid B was resuspended in resuspension buffer consisting of 25 mM Tris-HCl (J. T. Baker, Phillipsburg, N.J.) at the pH of 8 and 10 mM Na2EDTA, to a final volume of 18.8 L. Plasmid B has a size of 4.7 kb. The resuspended cells were mixed with the lysis solution consisting of 0.2 N NaOH (J. T. Baker, Phillipsburg, N.J.) and 1% SDS (J. T. Baker, Phillipsburg, N.J.) at an equal flow rate of 300 mL / min by a Silverson Model L4R rotor / stator mixer, which was operated at a rotor speed of 800 rpm. The lysate effluent from mixer retained 5-minute holding time in the holding coil before entering the bubble column to mix with the pre-chilled (4-5° C.) neutralization / precipitation (NP) solution. The NP solution containing 1 M potassium acetate (J. T. Baker, Phillipsburg, N.J.) and 3 M ammonium acetate (EMD Chemicals, Inc., Bibbstown, N.J.) was fed to the bubble column mixer at 600 mL / min, simultaneously, the compressed air was introduced f...
example 3
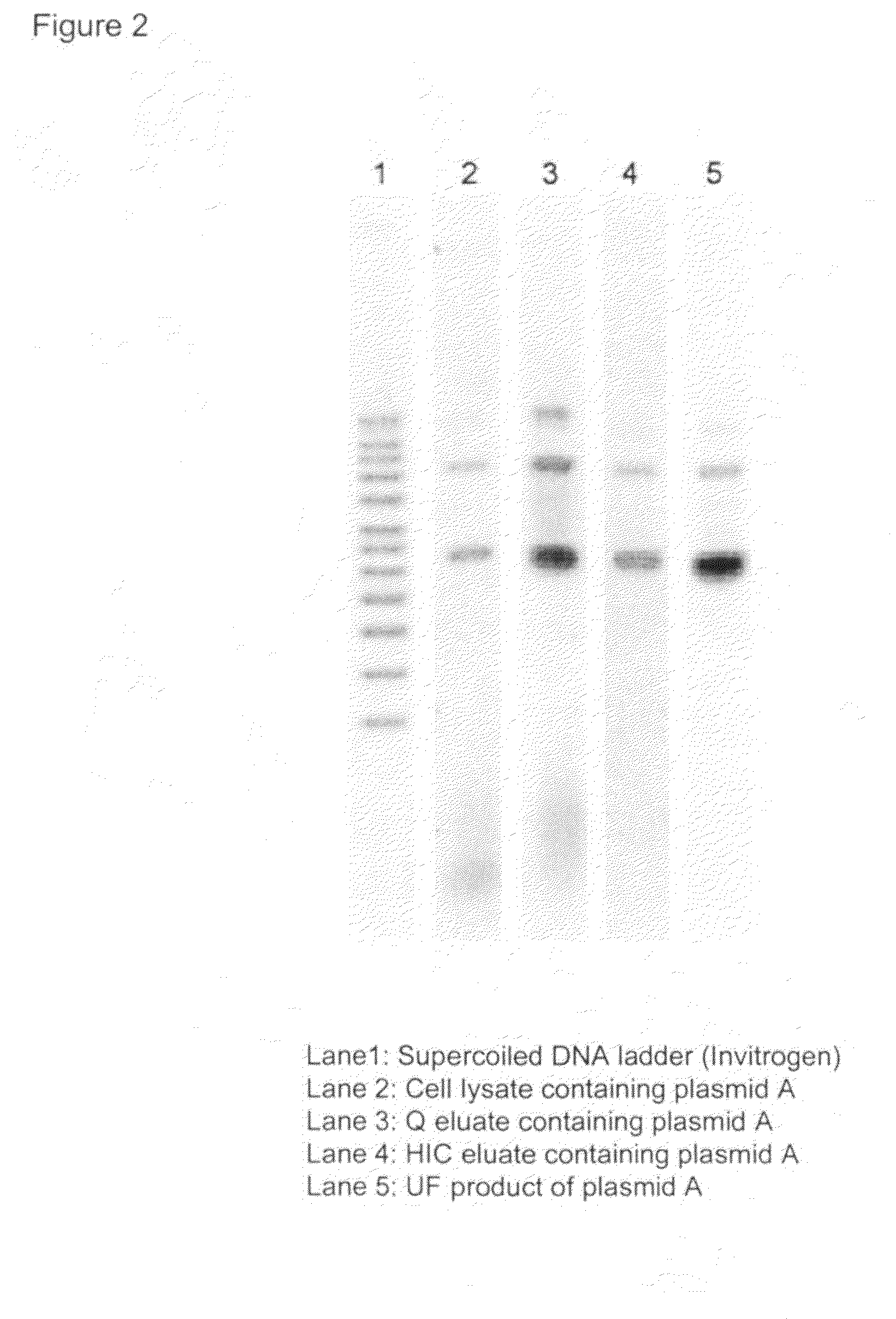
[0093]E. coli cells containing a plasmid C (plasmid size of 3.5 kb) were fermented to high cell density and harvested by centrifugation. A wet cell weight of 24.4 kg cells were recovered from 400-L working volume of fermentation. The cells were resuspended with 171 L of resuspension buffer consisting of 25 mM Tris-HCl (J. T. Baker, Phillipsburg, N.J.) at the pH of 8 and 10 mM Na2EDTA (Mallinckrodt Baker, Phillipsburg, N.J.) for a period of 3 hours. The resuspended cells were mixed with fresh lysis solution by Silverson high shear mixer at the same flow rate of 600 ml / min, and held for approximately 5 min accomplished by a holding loop. The neutralization / precipitation solution consisting of 1 M potassium acetate (J. T. Baker, Phillipsburg, N.J.) and 3 M ammonium acetate (EMD Chemicals, Inc., Bibbstown, N.J.) were pumped to the bubble mixer at a flow rate of 1.2 L / min to mix with lysed cells. Compressed air fed at a gas flow rate of 5-6 slpm served as the mixing force and transportin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com