Process for producing optically active ester
a technology of optically active and ester, which is applied in the preparation of carboxylic acid esters, chemistry apparatus and processes, and organic chemistry, etc. it can solve the problems of not being considered an industrially applicable process, not being suited to the production of high-optical purity 2-hydroxybutyric, and being very expensive products. , to achieve the effect of high yield and high optical yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of n-butyl(S)-2-hydroxy-(S)-3-phenylthiobutyrate
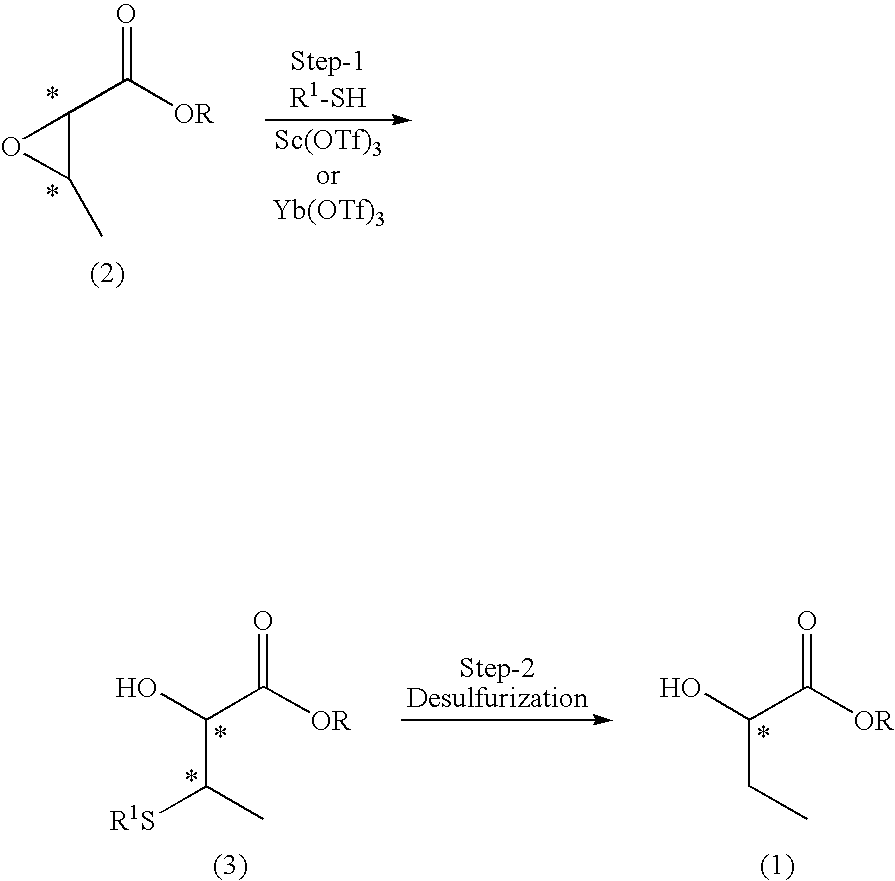
[0043]Scandium trifluoromethanesulfonate [Sc(OTf)3] (311 mg) and thiophenol (0.064 mL) were sequentially added at room temperature to a solution of n-butyl (2S,3R)-2,3-epoxybutyrate (100 mg, 97.3% ee) (product of Osaka Organic Chemical Industry, Ltd.) in methylene chloride (5.0 mL), and the mixture was stirred at the same temperature for 10 hours. The reaction mixture was added to water (20 mL), and extracted with chloroform (20 mL×3). The organic layers were combined, and the combined organic layer was dried over sodium sulfate anhydrate and concentrated under reduced pressure. The residue was purified through silica gel column chromatography (n-hexane / ethyl acetate=5 / 1), to thereby yield colorless oil (117 mg, 95.3%).
[0044]IR(ATR); 3484, 2961, 2933, 2873, 1732, 1645, 1474, 1439, 1380, 1240, 1211, 1129, 1090, 1051, 1024, 951, 747, 692 cm−1.
[0045]1H-NMR (400 MHz, CDCl3) δ; 0.94 (t, J=7 Hz, 3H), 1.27 (d, J=7 Hz, 3H) 1.38 (sext...
example 2
Synthesis of n-butyl(S)-2-hydroxy-(S)-3-phenylthiobutyrate
[0048]Ytterbium trifluoromethanesulfonate [Yb(OTf)3] (196 mg) and thiophenol (0.032 mL) were sequentially added at room temperature to a solution of n-butyl (2S,3R)-2,3-epoxybutyrate (50 mg) in methylene chloride (3.0 mL), and the mixture was stirred at the same temperature for 24 hours. The reaction mixture was added to water (20 mL), and extracted with chloroform (20 mL×3). The organic layers were combined, and the combined organic layer was dried over sodium sulfate anhydrate and concentrated under reduced pressure. The residue was purified through preparative thin-layer chromatography (n-hexane / ethyl acetate=5 / 1), to thereby yield colorless oil (69 mg, 81.3%).
example 3
Synthesis of n-butyl(S)-2-hydroxy-(S)-3-ethylthiobutyrate
[0049]Scandium trifluoromethanesulfonate [Sc(OTf)3] (311 mg) and ethanethiol (0.047 mL) were sequentially added at room temperature to a solution of n-butyl (2S,3R)-2,3-epoxybutyrate (100 mg) in methylene chloride (5.0 mL), and the mixture was stirred at the same temperature for 7 hours. The reaction mixture was added to water (20 mL), and extracted with chloroform (20 mL×3). The organic layers were combined, and the combined organic layer was dried over anhydrous sodium sulfate and concentrated under reduced pressure. The residue was purified through silica gel column chromatography (n-hexane / ethyl acetate=5 / 1), to thereby yield colorless oil (116 mg, 83.4%).
[0050]IR(ATR); 3490, 2962, 2931, 2873, 1732, 1456, 1379, 1260, 1239, 1209, 1130, 1088, 1060, 980, 784, 766, 738, 699 cm−1.
[0051]1H-NMR (400 MHz, CDCl3) δ; 0.95 (t, J=7 Hz, 3H), 1.24 (d, J=7 Hz, 3H), 1.28 (t, J=7 Hz, 3H), 1.40 (sext, J=7 Hz, 2H), 1.63-1.70 (m, 2H), 2.57-2....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com