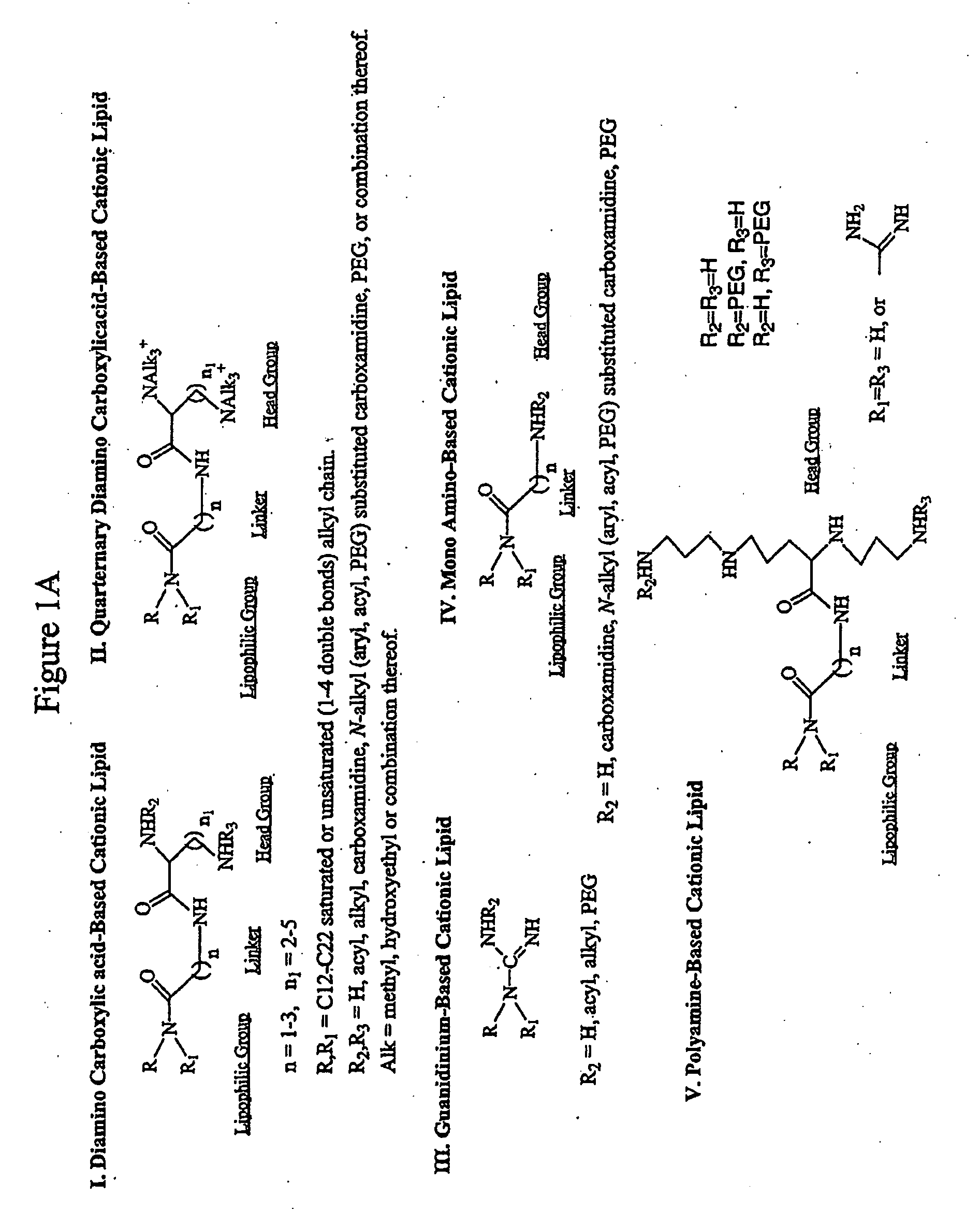
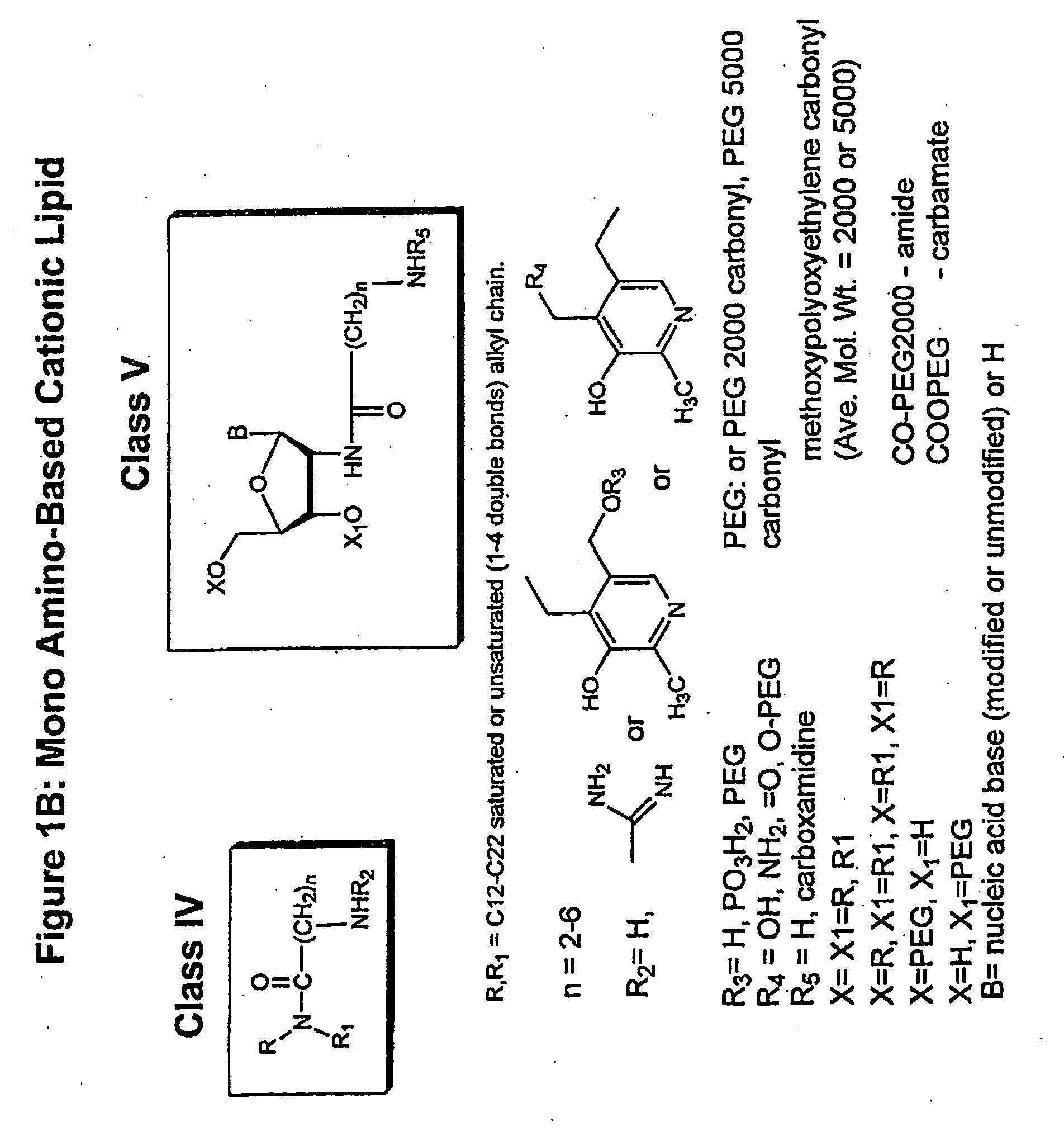
Novel Compositions for the Delivery of Negatively Charged Molecules
a technology of negatively charged molecules and compositions, applied in the direction of organic chemistry, sugar derivatives, steroids, etc., can solve the problems of high restriction of charged molecules into living cells, inability to introduce chemically synthesized molecules into cells, and limited utility, so as to facilitate the diffusion of the enhancer:polymer complex
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Diaminobutyric Acid and Guanidinium-Based Cationic Lipids (FIG. 2)
[0139]Synthesis of palmityloleylamine (1): 1-bromohexadecane (15.27 g, 50 mmol) was rapidly added to oleylamine (26.75 g, 100 mmol) at 100° C. The reaction mixture was heated at 120° C. for 30 minutes and than cooled to room temperature. Chloroform (200 ml) was added followed by 1 N NaOH (50 ml). The mixture was then extracted with H2O (200 ml), the organic layer dried (Na2SO4) and concentrated to a syrup. Silica gel column chromatography using 5-20% gradient methanol in dichloromethane afforded 20.5 g of palmityloleylamine as a syrup (yield, 83%). The identity of the product was confirmed using NMR spectroscopy. 1H NMR (CDCl3) d 5.34 (m, 2H, CH═CH), 2.58 (m, 4H), 2.00 (m, 4H), 1.47 (m, 4H), 1.25 (m, 48H), 0.86 (m, 6H). FAB-MS: 493 [M+H]+. (Other reagents could include oleyl-bromide and hexadecane amine)
[0140]Synthesis of N′-palmityl-N′-oleyl-N-CBZ-glycinamide (2): (1) (2.46 g, 5 mmol) was added to a solu...
example 2
Synthesis of DS 46596 (12)
[0148]Synthesis of Cholesterylamine (10): Referring to FIG. 3, cholesteryl chloride (10 grams, 25 mmol) was partially dissolved in dry methanol (50 ml) and the solution was heated with stirring to 155° C. for 18 hr at 500 psig using a 300 ml Parr bomb apparatus charged with dry ammonia gas. The bomb was cooled to room temperature and the methanol was removed by steam distillation on a rotary evaporator. Compound 10 was purified using E. Merck silica chromatography by eluting with dichloromethane / methanol (4:1 v / v) to yield 4 grams of the ninhydrin positive product (yield, 60%). Identity was confirmed by ES-MS.
[0149]Synthesis of Boc3arginineNHcholesterylamide (11): A 200 mL pear shaped flask with stir bar was charged with a mixture of 10 (1 g, 2.6 mmol), Boc3 arginine (1.2 grams, 2.6 mmol), diisopropylcarbodiimide (450 ul, 2.9 mmol) and dichloromethane (70 mls). The mixture of reagents was stirred at room temperature for two hours. Following the reaction, th...
example 3
Synthesis of PH 55933 (15) and PH 55938 (16)
[0151]Synthesis of (13): Referring to the FIG. 4, to a solution of methylphosphonic dichloride (0.332 g, 2.5 mmol, 31P NMR s, 43.93 ppm) stirring at room temperature under positive pressure argon was added 4-dimethylaminopyridine (DMAP) (0.31 g, 2.5 mmol). The resulting clear, colorless solution was cooled to −70° C. and a solution of cholesterol (0.97 g, 2.5 mmol) suspended in anhydrous dichloromethane (20 ml) was added via syringe with vigorous stirring over a period of one hour. The reaction mixture was allowed to warm to room temperature and was maintained at room temperature for 18 hours at which time 31P NMR analysis of a small aliquot of the reaction mixture indicated complete reaction (d, 39.08 ppm).
[0152]Crude (13) was treated with additional DMAP (0.31 g, 2.5 mmol) and the reaction mixture cooled to −70° C. while stirring under positive pressure argon. H-Lys(Z)-OCH3 (0.66 g, 2.25 mmol) in anhydrous dichloromethane (20 ml) was add...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com