A method for preparing a particulate cathode material, and the material obtained by said method
a cathode material and cathode material technology, applied in the field of preparing a particulate cathode material and the material obtained by said method, can solve the problems of low electronic conductivity of cathode materials, good attachment, and covalent bonding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis
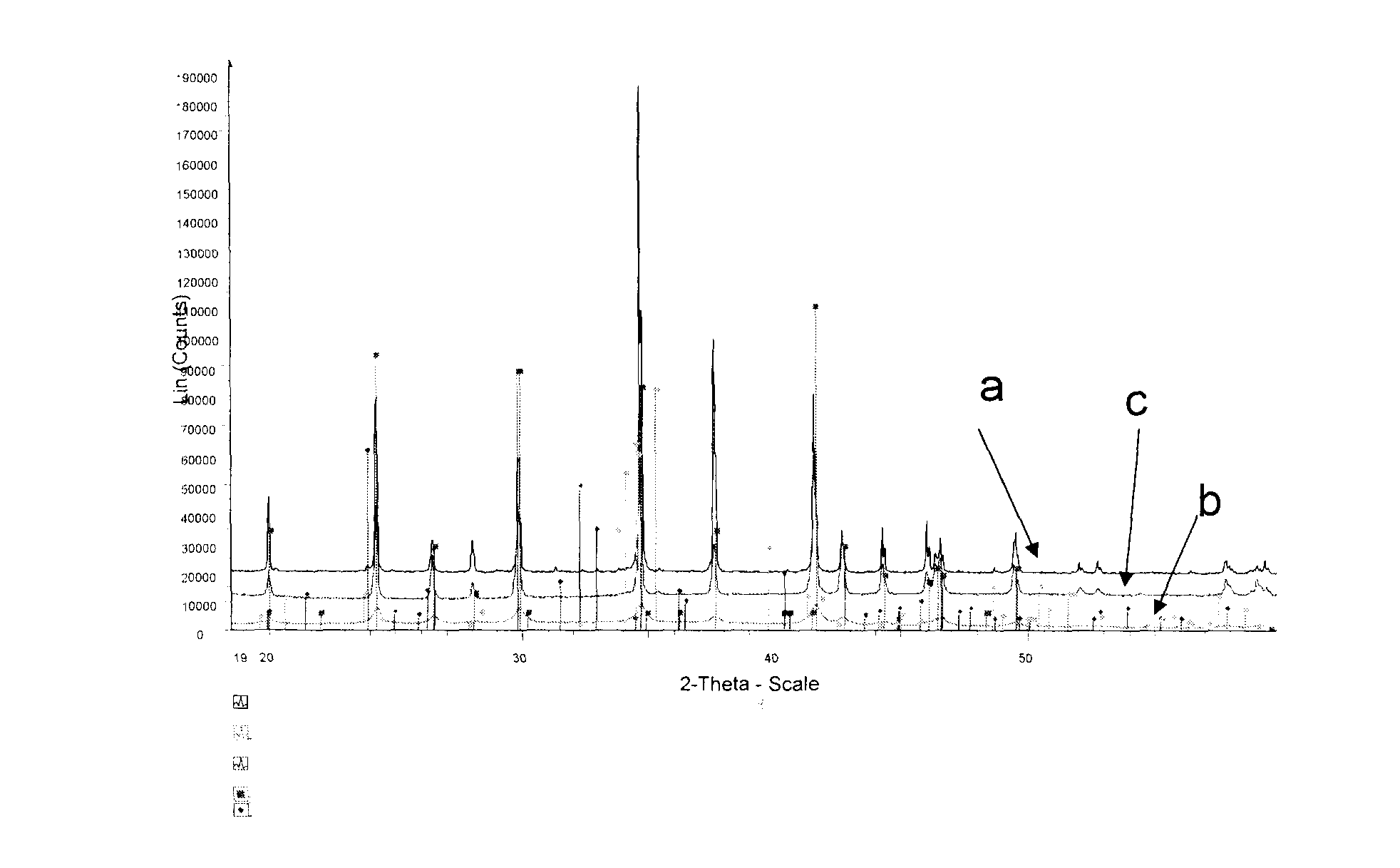
[0133]In first step, LiFePO4 was synthesized by melt casting using the process described in WO05.062404. 2 FePO4.2H2O and 1 Li2CO3 were mixed at nominal LiFePO4 composition with an excess of 0.5 mole of EBN-1010 (graphite powders), and then heated to 1050° C. in a graphite crucible under inert atmosphere in a furnace. The melt was held at 1050° C. for 1 h and then cooled down in the furnace. X-ray analysis has confirmed that the obtained ingot has a LiFePO4 main phase and minor Li4P2O7 and Fe2P2O7 phases as shown in curve a of FIG. 1. The impurity phase accounts for less than 3% of the total materials.
[0134]In a second step, the ingot was crashed into millimeter sized particles by using a jaw crusher with ceramic liner to avoid metal contamination. The millimeter sized particles are further ground by using ball milling to achieve micrometer sized particles. Finally, the micrometer sized powders were dispersed in IPA solution at 10-15% of solid concentration and then ground ...
example 2
[0143]A suspension in IPA of nanometer sized particles of LiFePO4 obtained after step 2 of Example 1 was dried at room temperature by blowing dry air. The obtained LiFePO4 was the re-dispersed in a water-lactose solution by using ultrasonic action. The ratio of lactose to LiFePO4 was 10 wt. %. After drying, lactose coated LiFePO4 particles are obtained.
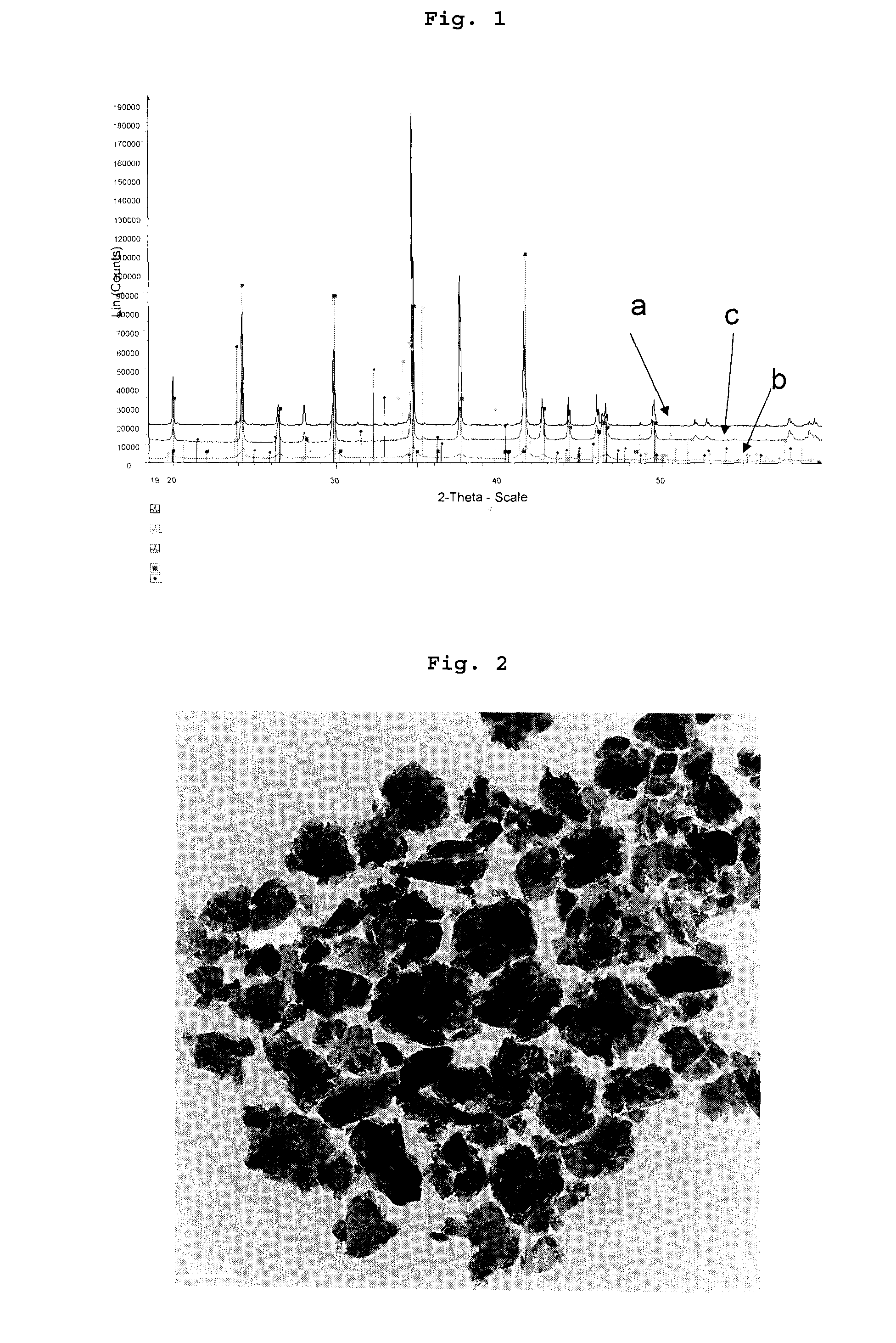
[0144]Thermal treatment LiFePO4 and carbonization of the lactose were performed in a rotary kiln as described in example 1. SEM and TEM observation have revealed that the nanoparticles obtained after thermal treatment are bigger when lactose is used as the carbon precursor, even starting from to same wet milled particle precursors.
example 3
[0145]The LiFePO4 was synthesized by a melt casting process and then milled to nanometer sized using a beads mill as described in example 1.
[0146]Poly(malic anhydride-1-alt-octadecene) was dissolved in IPA and then mixed with the LiFePO4 in an IPA suspension at a ratio of 5 wt %. After that, the solution was spray dried and spherical aggregates are obtained.
[0147]The spray dried aggregates containing LiFePO4 nanoparticles and Poly(malic anhydride-1-alt-octadecene) organic precursor were thermal treated in a rotary kiln as described in example 1.
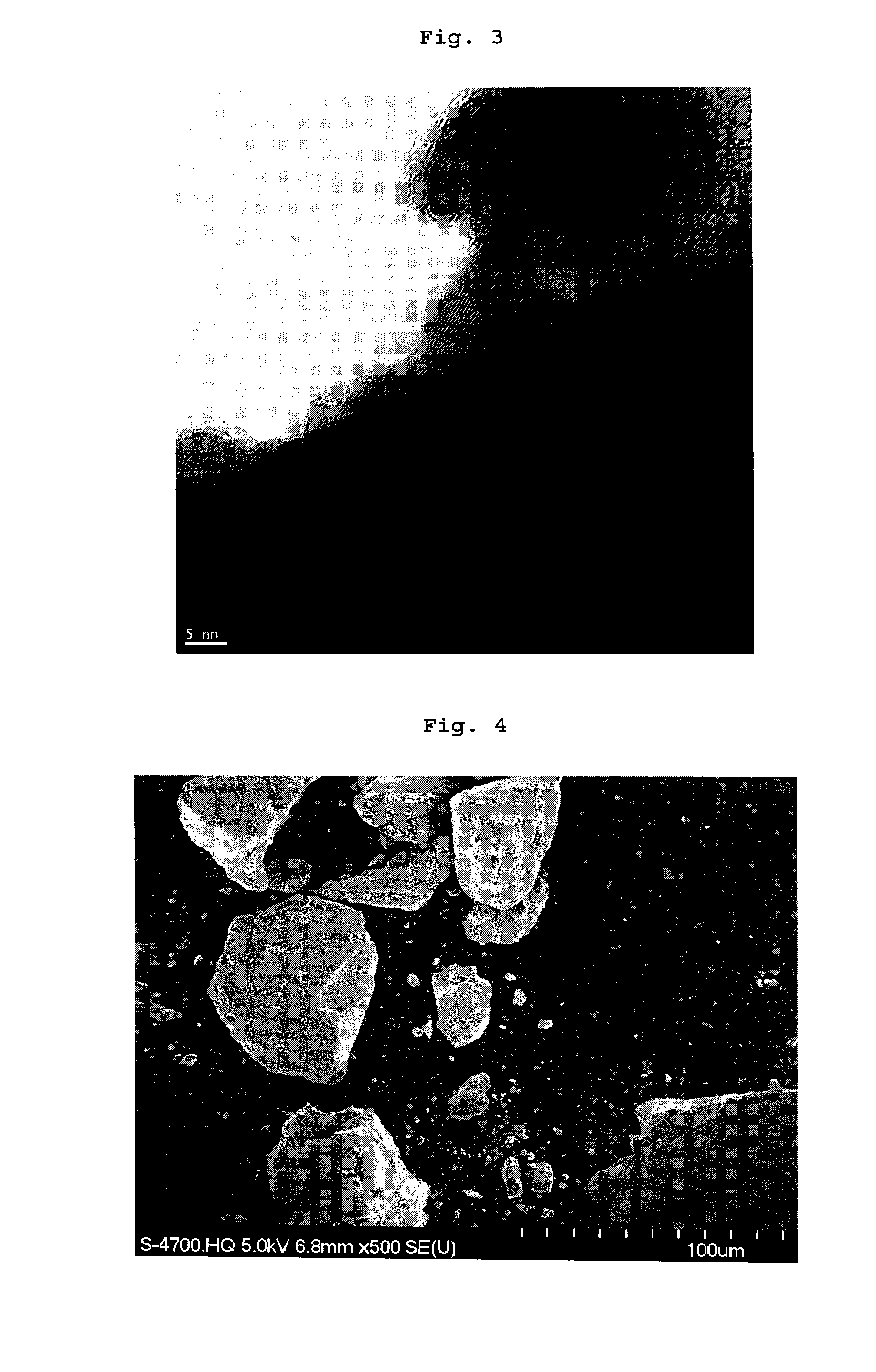
[0148]SEM analyses show that spherical micron sized C—LiFePO4 aggregates of nano particles are obtained (see FIGS. 7 & 8). A thin layer of conductive carbon is deposited on the surface of the nanoparticles.
PUM
| Property | Measurement | Unit |
|---|---|---|
| mean size diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| mol % | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com