Method To Synthesize Colloidal Iron Pyrite (FeS2) Nanocrystals And Fabricate Iron Pyrite Thin Film Solar Cells
a technology of colloidal iron pyrite and nanocrystals, which is applied in the field of solar cells and nanocrystal-based solar cell devices, can solve the problems of limited market share and societal impact of cdte and cigs, and the inability to meet the large increase in energy demand by existing carbon-based technologies without further destabilizing the clima
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
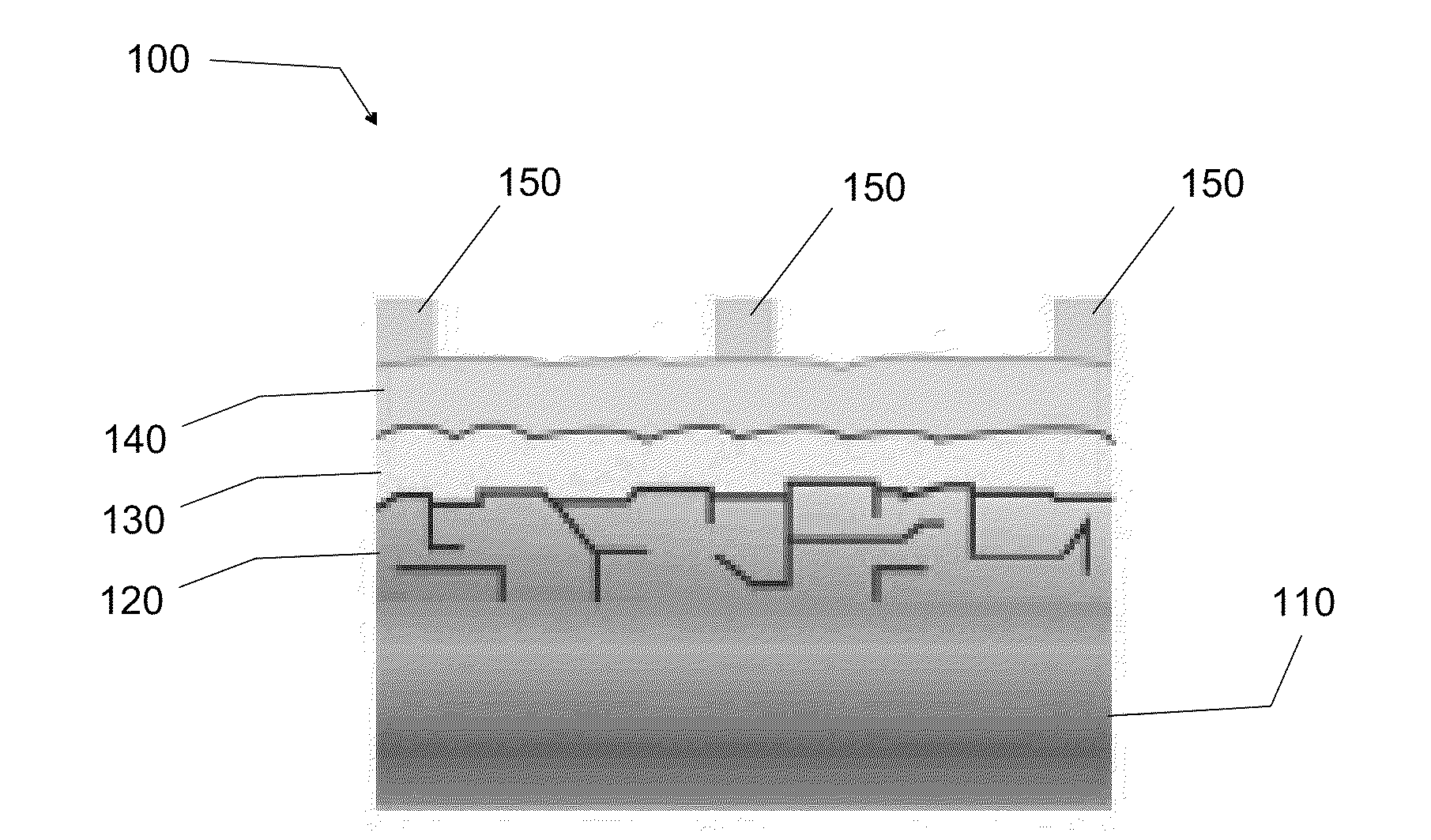
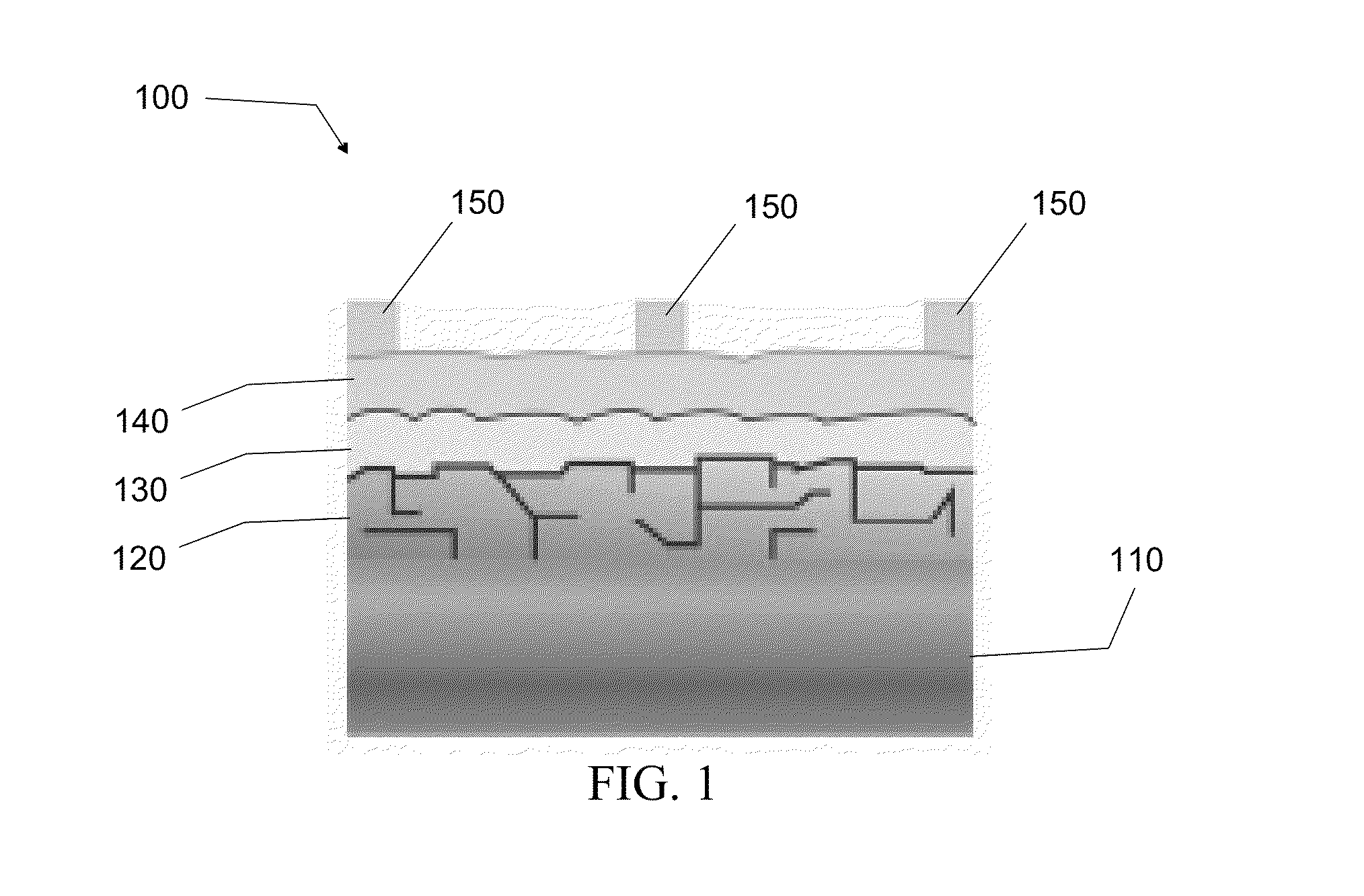
[0038]The first embodiment is directed to a method of synthesizing high-quality pyrite NC thin films from stable colloidal dispersions of single-crystalline, phase-pure pyrite NCs and then sintering the NC films in sulfur at moderate temperatures to produce large-grain polycrystalline pyrite films, which are promising for the p-type pyrite thin film 120 layer of p-n junction pyrite solar cell system 100.
[0039]Pyrite NCs are of particular interest in low-cost solar energy conversion because of the prospect of fabricating inexpensive, large-area photovoltaics by roll-to-roll deposition of NC solar ink or paint on flexible substrates. NC-based photovoltaics processed from solution may offer excellent manufacturing scalability at very low cost compared to conventional single crystal and thin film approaches.
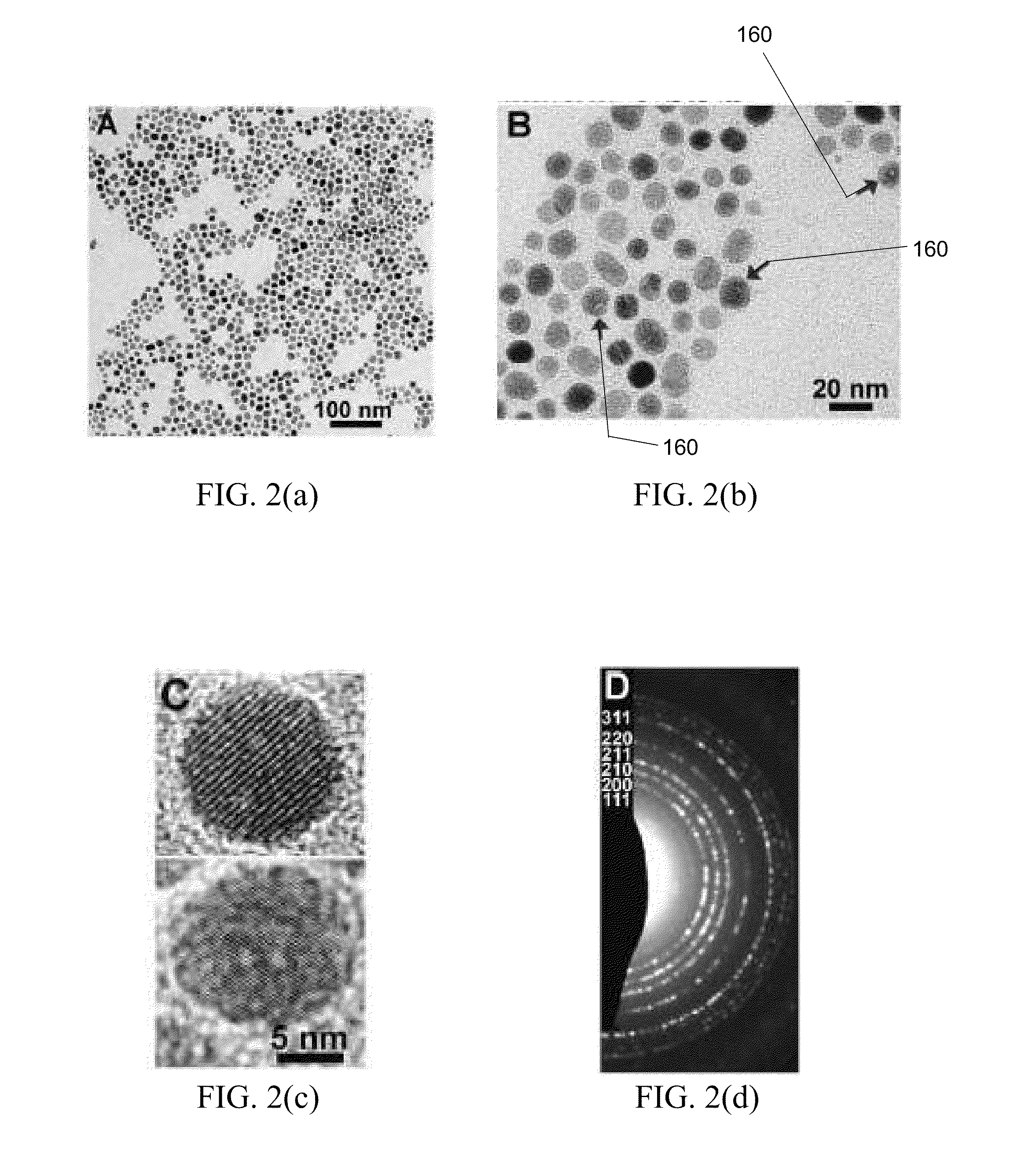
[0040]In one embodiment, colloidal pyrite NCs are synthesized by the following method. In a container, such as a three-neck flask, a reaction solution is created by mixing FeCl2.4H2O...
second embodiment
[0055]The second embodiment is directed to a method of fabricating p-type pyrite thin film 120 into high-quality, microcrystalline stoichiometric pyrite thin films by depositing microcrystalline pyrite onto substrate layer 110 by metal-organic chemical vapor deposition (MO-CVD). CVD is the best gas-phase process for depositing pyrite because it offers superior control of film morphology, purity and doping compared with alternative gas-phase methods (e.g., evaporation, sputtering, sulfurization of iron films, etc.). In a preferred embodiment, the substrate layer 110 is a metal foil. The use of a variety of different substrates, however, can serve as the substrate layer 110 in pyrite thin film 120. In particular, other flexible metal foil substrates (molybdenum, steel, aluminum, copper, etc.) that are electrically conductive can serve as the substrate layer 110 in p-type pyrite thin film 120. Other substrates such as glass and silicon can also serve as the substrate layer 110 in p-typ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| open-circuit voltage | aaaaa | aaaaa |
| diameters | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com