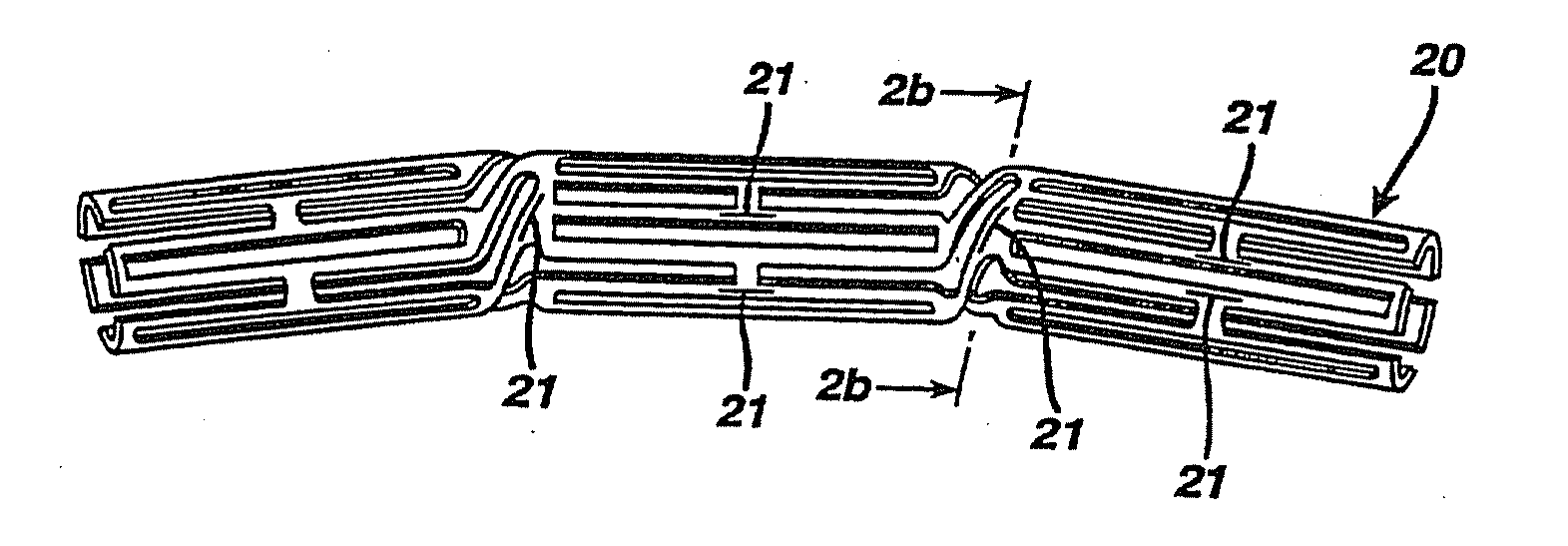
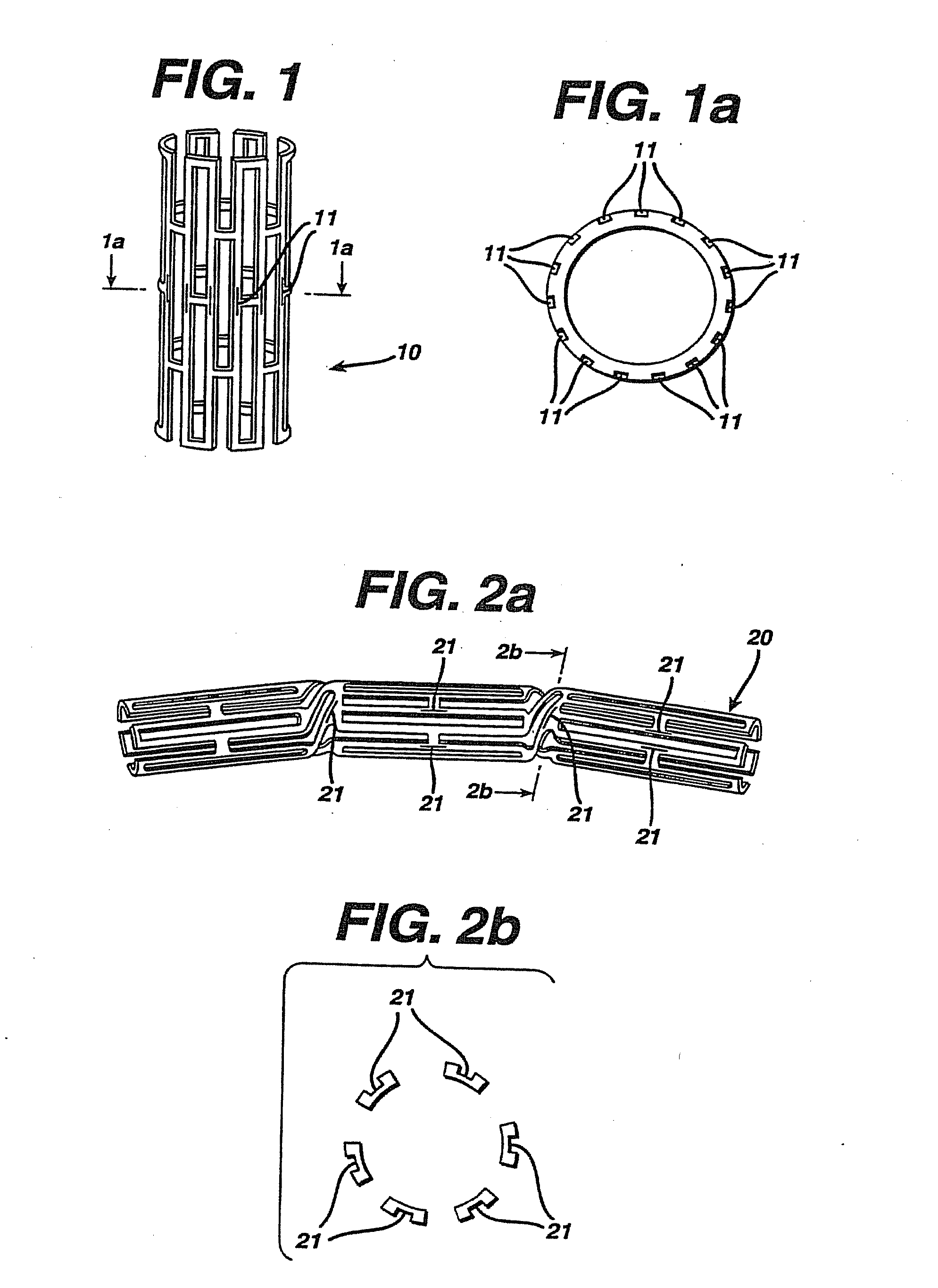
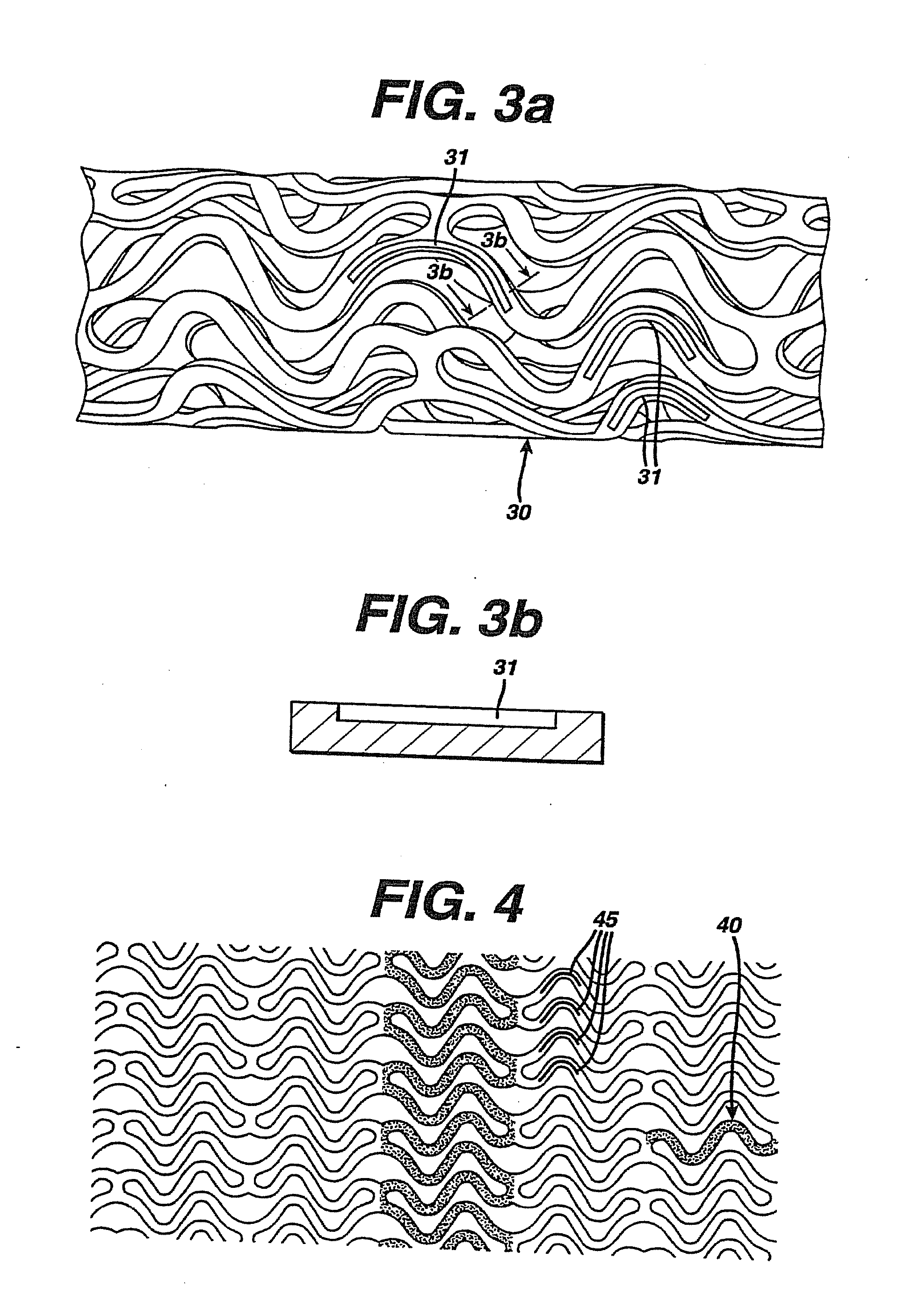
Drug Combinations Useful for Prevention of Restenosis
a technology of restnosis and drug combinations, applied in the direction of prosthesis, blood vessels, catheters, etc., can solve the problems of significant restnosis, post-ptca closure of the vessel, and 500,000-600,000 deaths annually, so as to reduce inflammation, improve the therapeutic effect, and reduce the proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0034]To assess the ability of a drug combination to prevent cell proliferation, human smooth muscle cells (Clonetics, Walkersville, Md.) were seeded at a density of 10,000 cells / well) into each well of 24-well plates and cultured in growth medium containing herapin, EGF (epidermal growth factor), FGF (fibroblast growth factor) and serum. After 24 hours, the growth medium was changed and fresh medium containing various concentrations of test agents (0.01-10 mcg / mL) were added to triplicate wells. Medium was replaced with fresh medium (plus test agents) after 3 days. On day five, cells were detached by trypsin / EDTA and counted using a hemacytometer. Cell viability was assessed by trypan blue exclusion.
[0035]Table 1 provides the percent of control growth of the various tested concentrations of the anti-inflammatory agent, dexamethasone, on human smooth muscle cells, either in the absence or presence of 2 concentrations of the antiproliferative / antiimmune agent, rapamycin. Dexamethason...
example 2
[0038]This example describes the preparation of a base coating that contains rapamycin.
[0039]Stents were coated with Parylene C™ using a vapor deposition method provided by the manufacturer of the parylene-coating instrument (SCS Madison, Wis.). The stent is weighed and then mounted for coating. While the stent is rotating a solution of 1.75 mg / ml Poly (ethylene-covinyl acetate) (PEVA), 1.75 mg / ml polybutyl methacrylate, and 1.5 mg / ml rapamycin dissolved in tetrahydrofuran is sprayed onto it. The coated stent is removed from the spray and allowed to air-dry. After a final weighing the amount of coating on the stent is determined.
example 3
[0040]This example describes the preparation of a base coating that contains dexamethasone.
[0041]Stents were coated with Parylene C™ using a vapor deposition method provided by the manufacturer of the parylene-coating instrument (SCS Madison, Wis.). The stent is weighed and then mounted for coating. While the stent is rotating a solution of 1.75 mg / ml Poly (ethylene-covinyl acetate) (PEVA), 1.75 mg / ml polybutyl methacrylate, and 1.5 mg / ml dexamethasone dissolved in tetrahydrofuran is sprayed onto it. The coated stent is removed from the spray and allowed to air-dry. After a final weighing the amount of coating on the stent is determined.
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com