Method for recovering elemental silicon from silicon sludge by electrolysis in non-aqueous electrolyte
a technology of elemental silicon and electrolysis, which is applied in the direction of electrolytic organic production, silicon compounds, instruments, etc., can solve the problems of large amount of carbon dioxide emitted, impurities contained, and limit the use of silicon sludge, so as to achieve the effect of efficient electrolysis of silicon
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Silicon Tetrachloride from Silicon Sludge
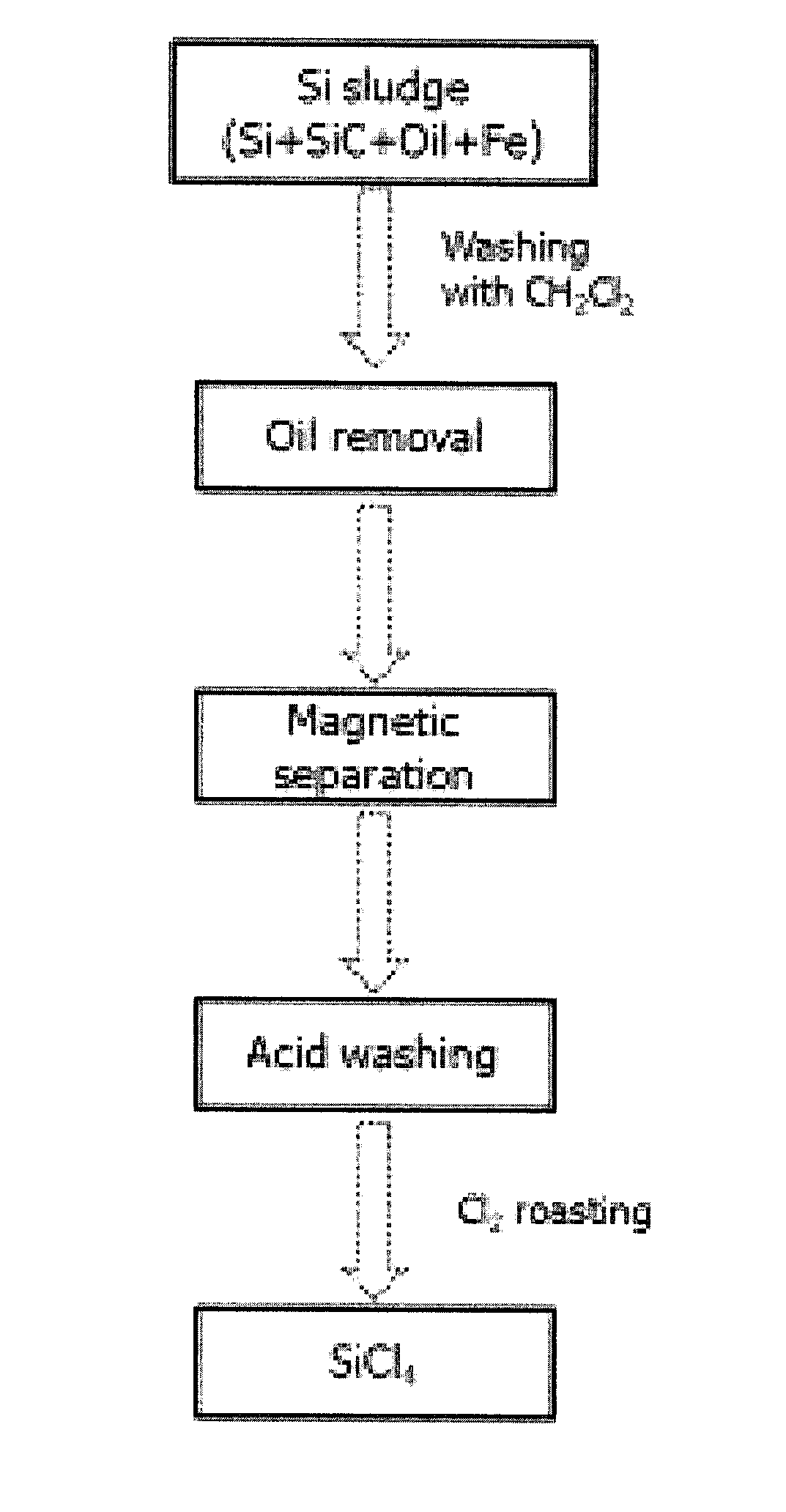
[0040]The outline of a process for preparing silicon tetrachloride as a pre-treatment for recovering silicon from silicon sludge is shown in FIG. 1.
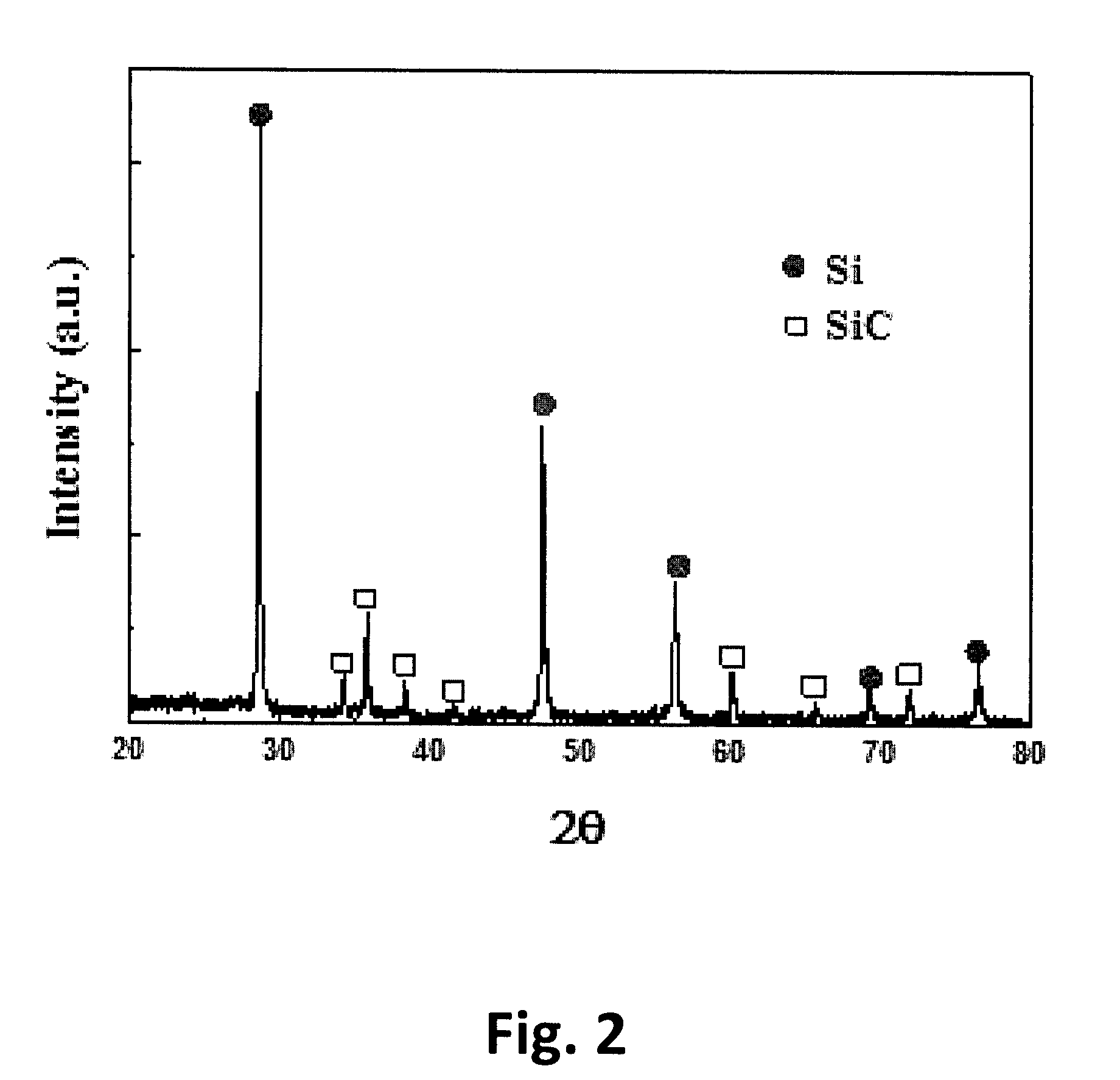
[0041]As an organic solvent miscible with cutting oil in silicon sludge, dichloromethane (CH2Cl2) was mixed with silicon sludge and stirred at 300 rpm or higher for 3 hours to selectively dissolve the cutting oil in the silicon sludge. After dissolving the cutting oil, silicon and silicon carbide (Si+SiC) as a solid phase and organic oil as a liquid phase were separated by filtration and centrifugation. Iron powder contained in the solid phase mixture, from which the organic oil is separated, was separated using a magnetic separator (Eric manufacturing) having a magnetic flux density of 500 gauss. The resulting mixture was stirred in 1 mol / L of hydrochloric acid solution at a solid-liquid ratio of 1:2 at room temperature for 2 hours, and the solid phase was precipitated, washed with...
example 2
Electrolytic Recovery of Silicon from Silicon Tetrachloride
[0044]1. Experimental Conditions for Electrolytic Recovery of Silicon
[0045]A three-electrode system was used as an electrolytic cell for electrolytic reduction of silicon. [EMIM]TFSI in which silicon tetrachloride was dissolved was used as an electrolyte. To prevent oxidation of silicon, the experiment was performed in a glove box in which high-purity argon gas (5 N, oxygen content of no more than 1 ppm, and vapor content of no more than 3 ppm) was filled. The effects of the stable voltage window of an ionic liquid and the electrolysis conditions (electrodes, ionic liquid, silicon concentration, etc.) on the electrochemical properties were measured by cyclic voltammetry using a Potentiostat / Galvanostat (Solartron 1287). At this time, the potential range was −4 to 2 V (vs. OCV), and the scan rate was 10 mV / s.
[0046]The electrochemical oxidation / reduction behaviors of silicon when gold (Ag) was used as a working electrode were ...
experimental example 1
Analysis of Silicon Electrodeposited Film
[0050]1. Analysis Method
[0051]The morphology and composition of the electrodeposited silicon film were analyzed using a field-emission scanning electron microscope (FE-SEM, JSM-6500F) with an energy-dispersive spectrometer (EDS) attached and X-ray diffractometer (XRD). Impurities in the electrodeposited silicon film were analyzed using X-ray photoelectron spectroscopy (XPS, ULVAC-PHI, Quantera SXM), and a depth analysis for a depth of about 50 nm from the surface was performed along with surface analysis. Moreover, the crystallinity of silicon was analyzed using X-ray diffractometer (XRD, Rigaku D / MAX-200-, Cu-Ka).
[0052]2. Analysis Results of Electrodeposited Film
[0053]The results of SEM-EDS of silicon reduced in a gold electrode are shown in FIG. 4.
[0054]As shown in FIG. 4, the morphology of silicon is in the form of particles of about 100 nm, and the results of the EDS analysis show the silicon reduced along with gold as the working electro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| magnetic flux density | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com