Bowel cleansing composition
a technology of bowel cleansing and composition, applied in the direction of drug compositions, antibacterial agents, aerosol delivery, etc., can solve the problems of patient's refusal or avoiding examination, inadequate bowel cleansing, refusal of repeat examination, etc., to achieve superior cleansing effect, enhance administration and compliance, and reduce volume
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
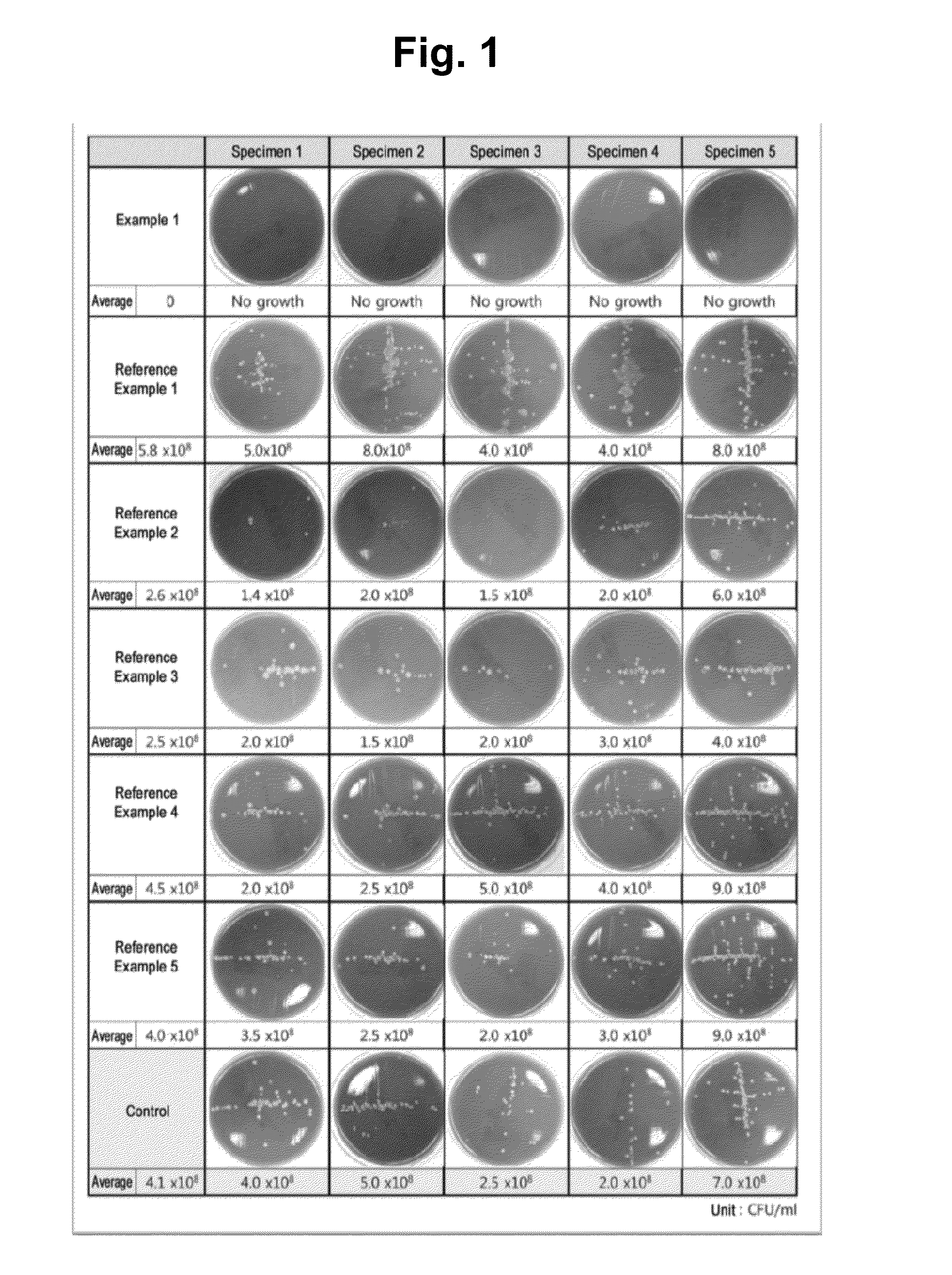
example 1
[0086]In 100 mL of carbonated water as solvent, 46 g of ascorbic acid, 2 g of calcium ascorbate, 40 g of sorbitol, 10 mg of bisacodyl, 1.5 g of citric acid, 33.5 mg of docusate sodium, 20 mg of caffeine, 100 mg of pectin, 30 mg of zinc oxide, 300 mg of simethicone, 1.5 g of sodium bicarbonate, 2 g of potassium bicarbonate, and 35 mg of sucralose were mixed to form 150 ml of a highly concentrated solution, and 350 ml of carbonated water was used as a separate vehicle to prepare a two-part bowel cleansing preparation.
[0087]Mixing was conducted by mixing all the ingredients in powder form at the same time and then pouring carbonated water to dissolve them. Caution is needed because a large amount of carbon dioxide (CO2) gas generated by the neutralization reaction between the acidic ascorbic acid and citric acid and basic bicarbonate salt causes extensive foaming Preparing a 1:10 diluted solution of Gasocol® containing the anti-foaming agent simethicone beforehand and adding it suitabl...
example 2
[0089]In 100 mL of bottled water as solvent, 30 g of ascorbic acid, 60 g of xylitol, 10 mg of bisacodyl, 1.5 g of citric acid, 300 mg of simethicone, 5 g of potassium bicarbonate, and 35 mg of sucralose were mixed to form 150 ml of a highly concentrated solution, and 350 ml of bottled water was used as a separate vehicle to prepare a two-part bowel cleansing preparation. Mixing of the ingredients was conducted in the same manner as in Example 1.
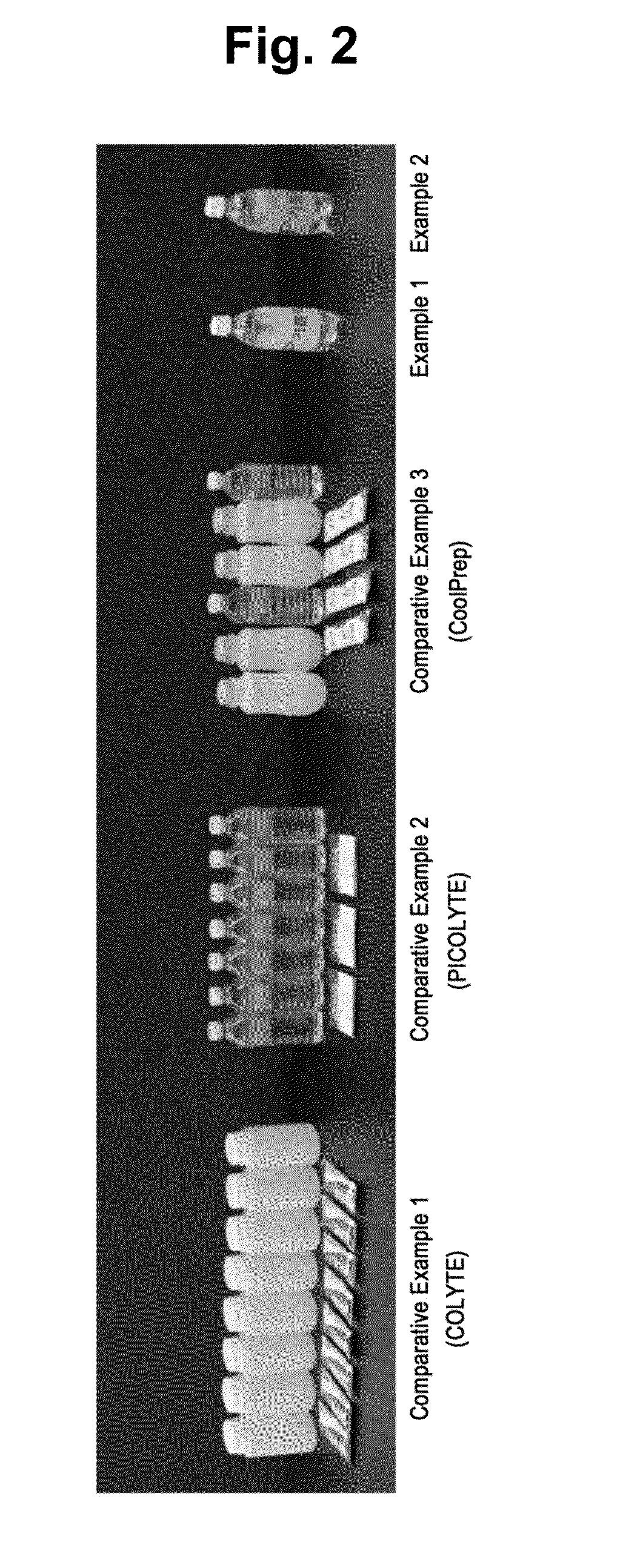
reference examples 1 to 6
[0090]For in vitro tests to assess the safety of sugar alcohols used as a first cleansing ingredient in the present invention, Reference Examples 1 to 6 were prepared for comparison with the solution of Example 1.
[0091]Reference Examples 1 to 6 refer to a COLYTE solution, a PICOLIGHT solution, a CoolPrep solution, a sorbitol (100 g / L) solution, a mannitol (100 g / L) solution, and a 12% xylitol (120 g / L) solution, respectively, with the COLYTE solution, PICOLIGHT solution, CoolPrep solution prepared by diluting the respective active ingredients in bottled water according to the manufacturer's instructions such that the concentration of each active ingredient is the same as its concentration used in the bowel preparation.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com