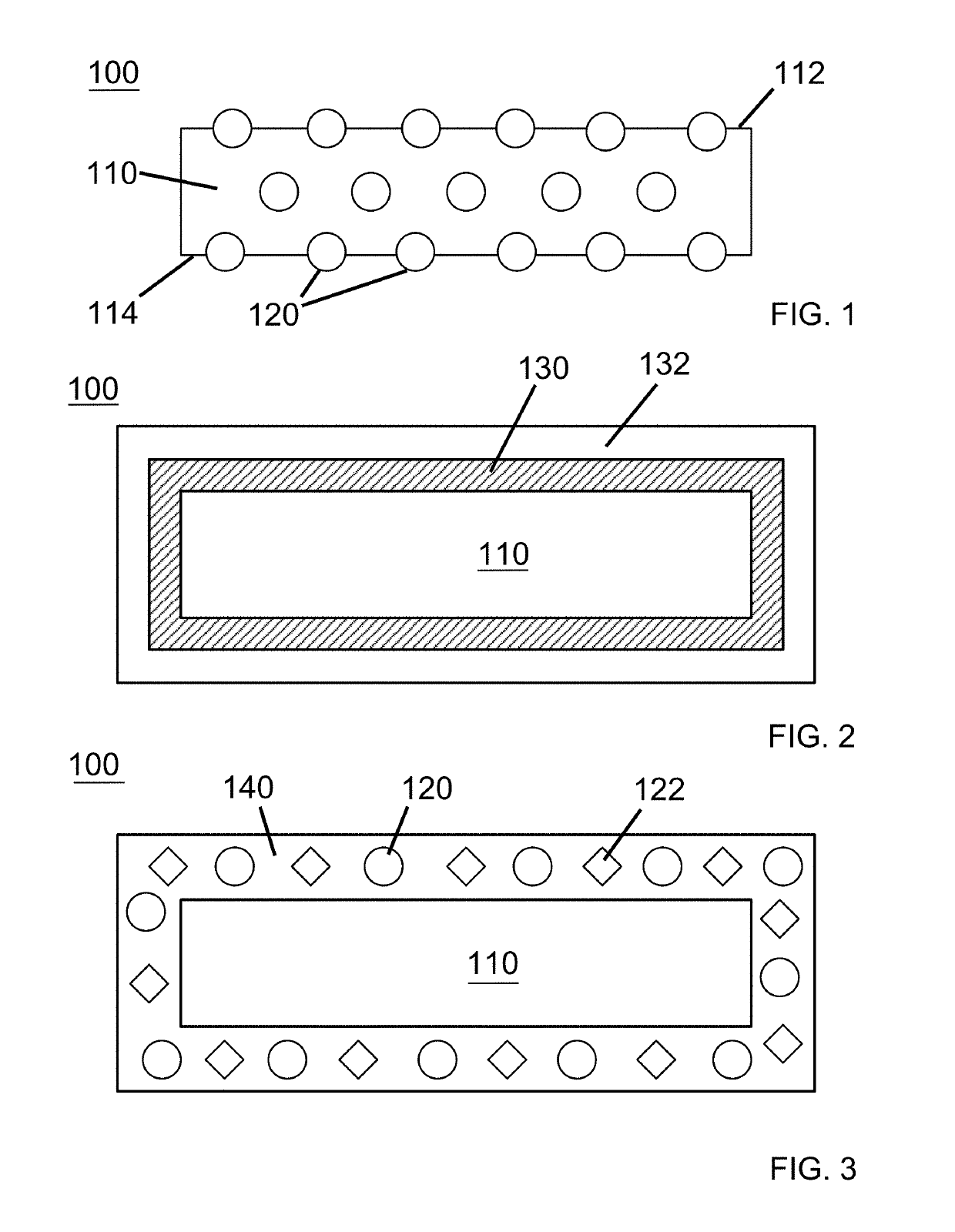
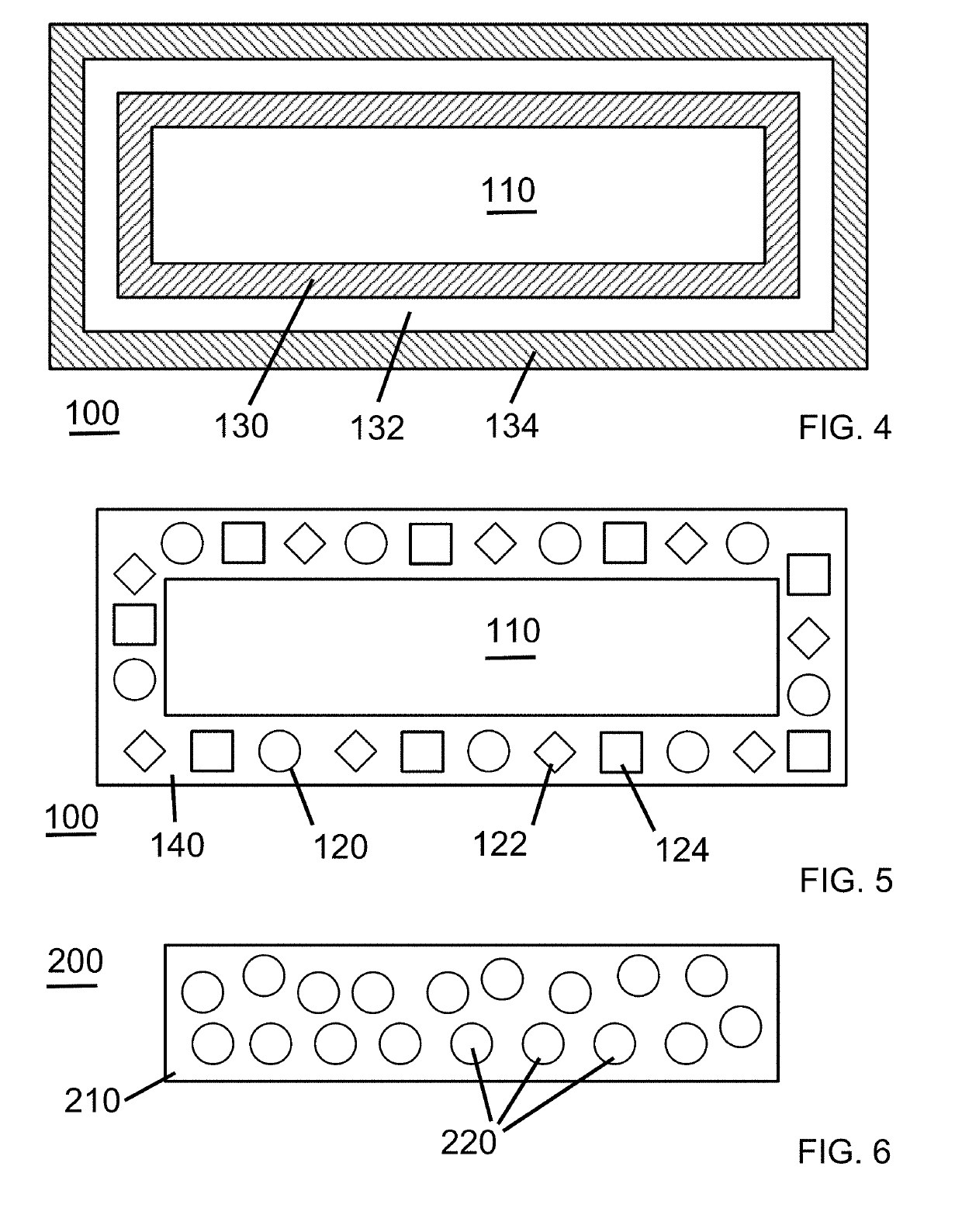
Dressings comprising platelet lysate
a technology of platelet lysate and dressing, which is applied in the field of dressings, can solve the problems of high limb amputation rate in military personnel, inconvenient use of incendiary materials, and frequent contamination with soil or sewage, so as to improve antimicrobial activity, minimize fluid loss, and avoid bleeding.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
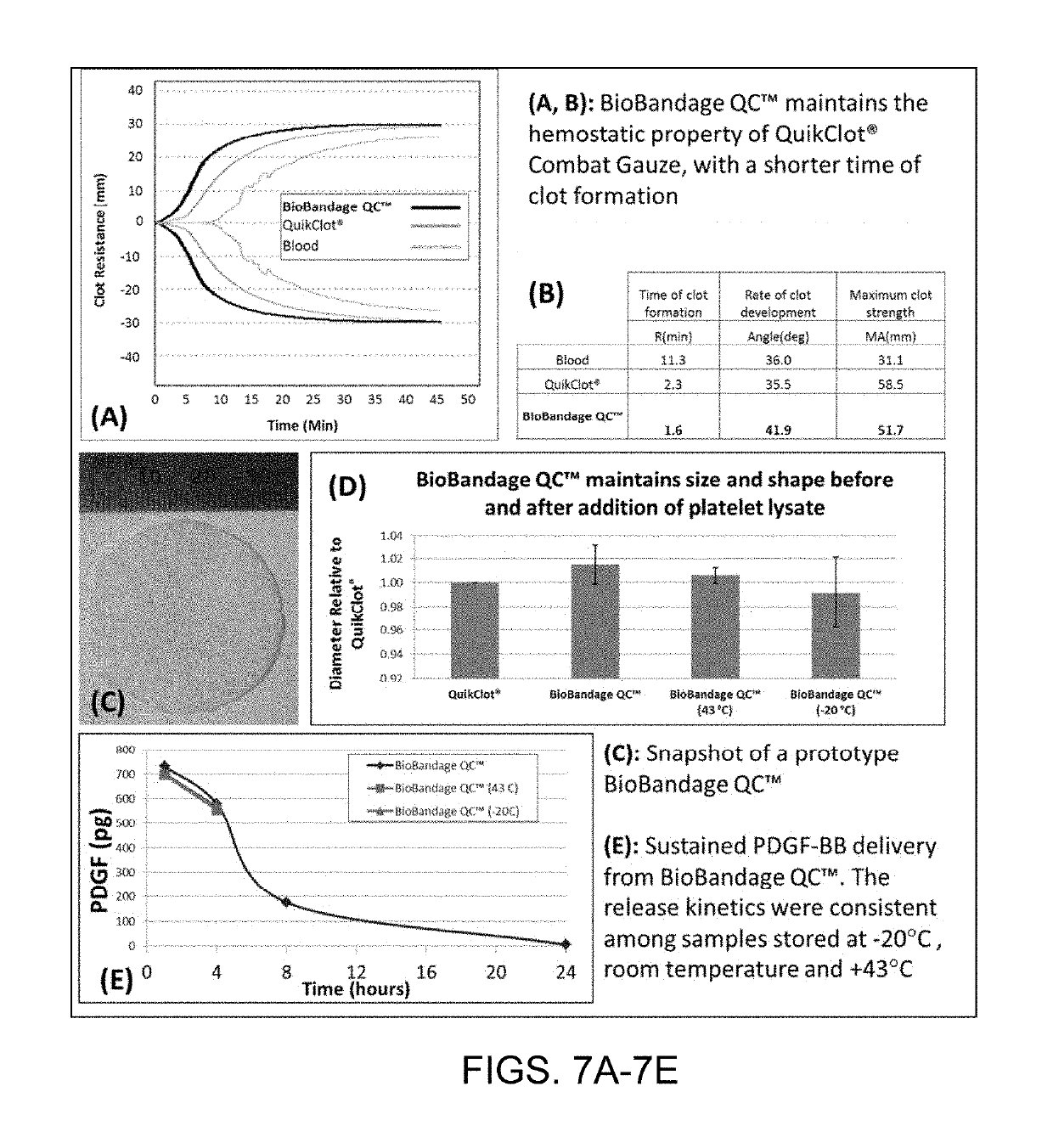
[0089]A preliminary bandage prototype was prepared by incubating 35 mm diameter circular disks of kaolin-impregnated gauze in human platelet lysate for 4 minutes at room temperature. The soaked gauze was immediately lyophilized. The initial in vitro hemostatic properties of the samples were measured by thromboelastography. The initial growth factor release characteristics of the samples were quantified using enzyme-linked immunosorbent assay (ELISA) with platelet-derived growth factor-BB (PDGF-BB), a pro-survival and angiogenic growth factor. The size, shape and mechanical strength of the samples before and after impregnation with hPL were also monitored.
[0090]FIGS. 7A-7E show the results. In these tests, blood was used as the control and baseline. “QuickClot®” refers to kaolin-impregnated gauze, and BioBandage QC™ refers to gauze that is impregnated with both kaolin and hPL. As seen in FIG. 7A and FIG. 7B, the addition of hPL did not reduce hemostatic capability. The BioBandage QC™...
example 2
[0091]To verify that lyophilized hPL still maintained growth factor activity, a cell culture supplement study was performed for three important dermal wound healing cell types: human mesenchymal stromal cells (MSCs), normal human dermal fibroblasts (NHDF), and neonatal human epidermal keratinocytes (NHEK). An aliquot of PLUS™ hPL was divided in two. One half of the aliquot was lyophilized and resuspended in an equal volume of deionized water, while the other half was frozen and then thawed prior to use. Culture media for each cell type was supplemented with both forms of 5% hPL as well as 10% FBS (as baseline) and the cells were cultured for a single passage. Culture with either form of PLUS™ resulted in no difference in the doubling times for all three cell types, and trended towards faster doubling times compared with FBS. These results indicate that the growth promoting activity of PLUS™ hPL was unaffected by the lyophilization process.
[0092]Next, to evaluate the ability of the l...
example 3
[0093]Another bandage prototype was prepared by combining bovine collagen and hPL at 37° C. for one hour, pouring the solution into molds, freezing the solution, and finally lyophilizing it. By weight, the bandage was approximately 27% collagen (1% w / v collagen solution) and 73% hPL. The resultant bandage was shelf stable. FIG. 9 is a picture of the bandage after being saturated with 0.9% saline, and shows the bandage is flexible and conformable to the surface of a wound.
[0094]The bandage demonstrated initial biocompatibility, safety, and non-toxicity testing. The bandage passed the following ISO 10993 tests: (A) Agar Diffusion cytotoxicity Test (mouse fibroblast L929 culture); (B) Kligman Maximization / Sensitization Test (guinea pigs); (C) Acute Systemic Injection Test (mice); (D) 28 Day Sub-Acute Systemic Toxicity by Implantation (rats); (E) 2 Week Intramuscular Implantation Test (rabbits); and (F) 4 Week Intramuscular Implantation Test (rabbits).
[0095]The bandage also demonstrated...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com