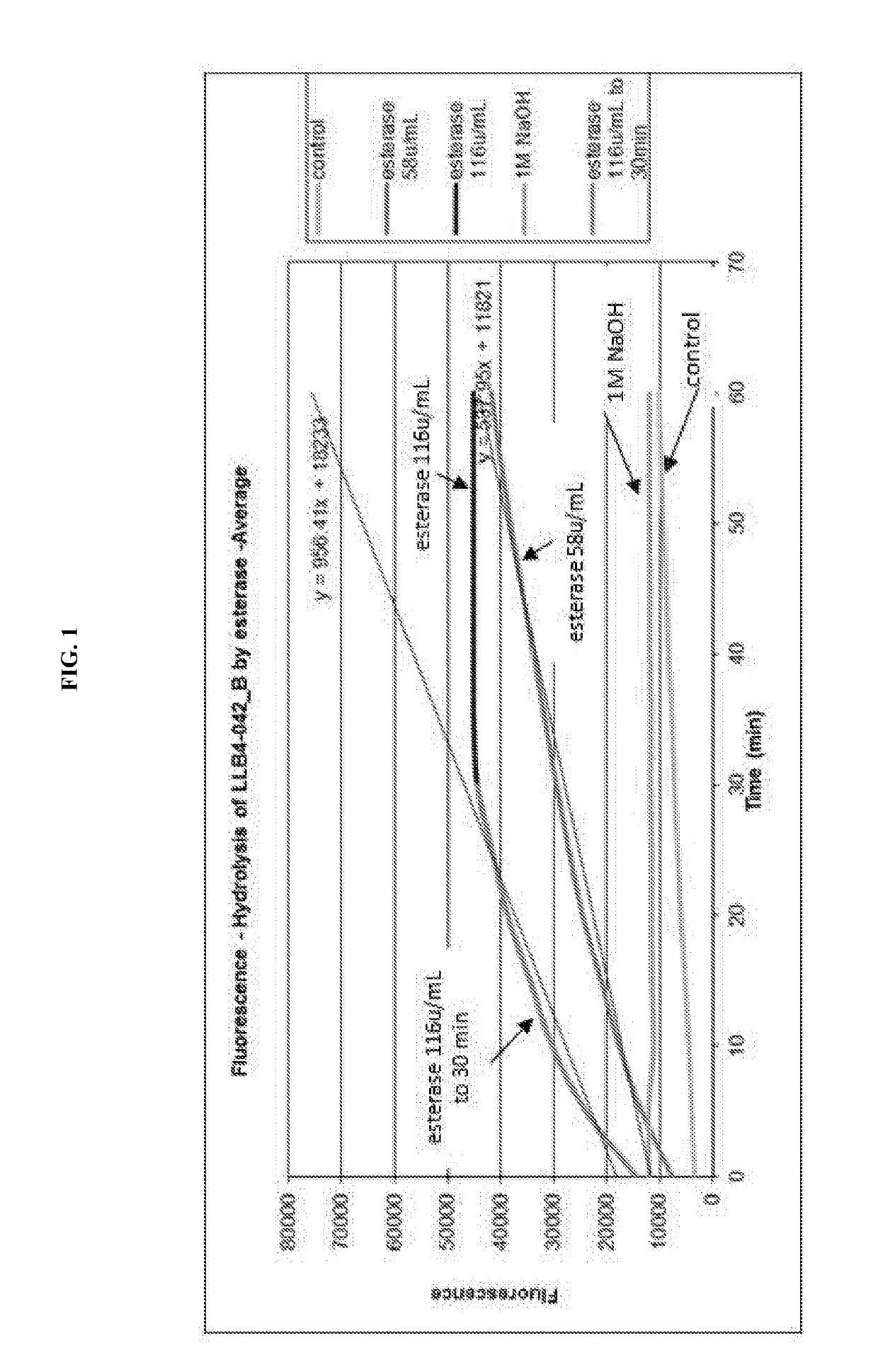
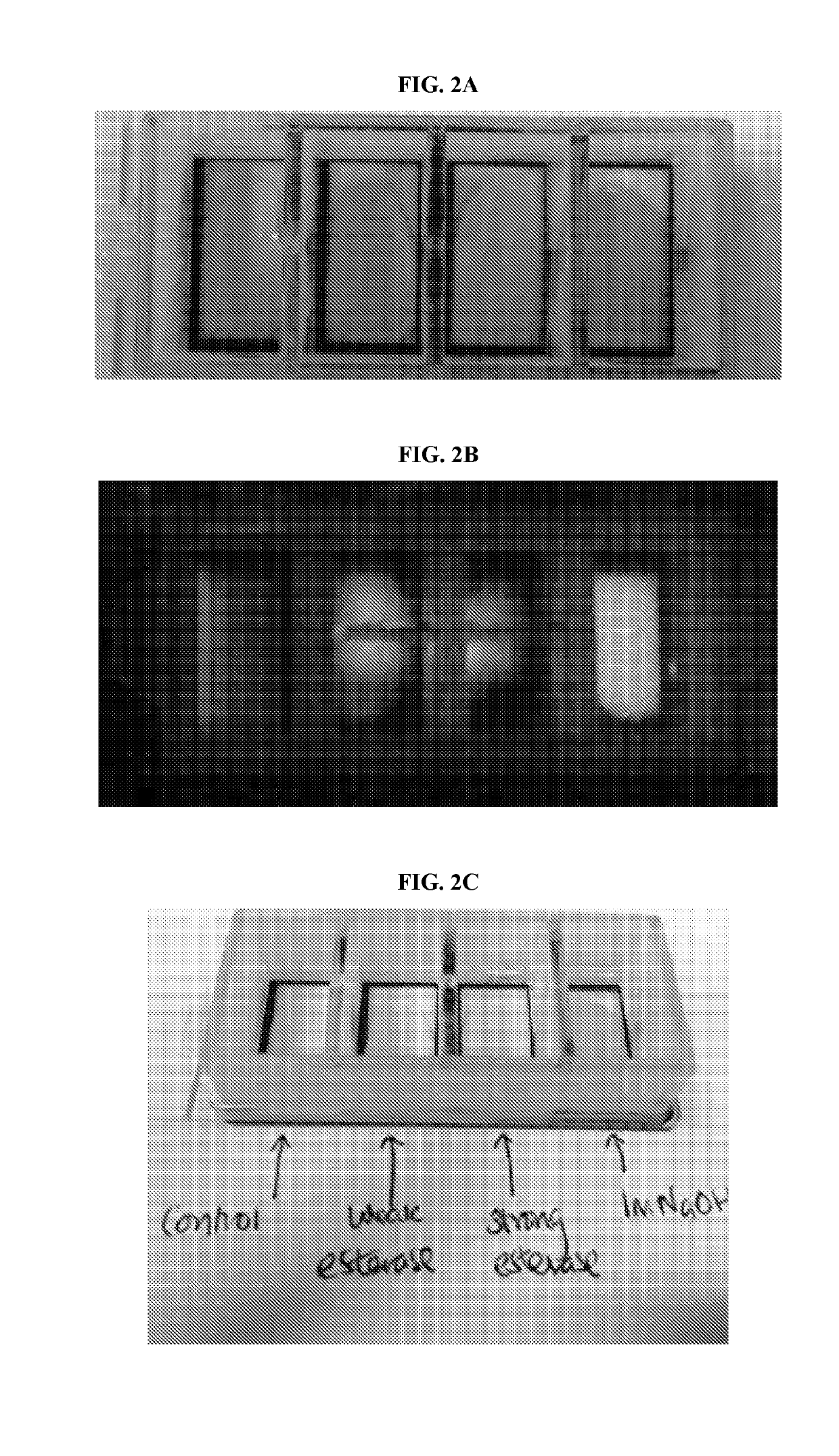
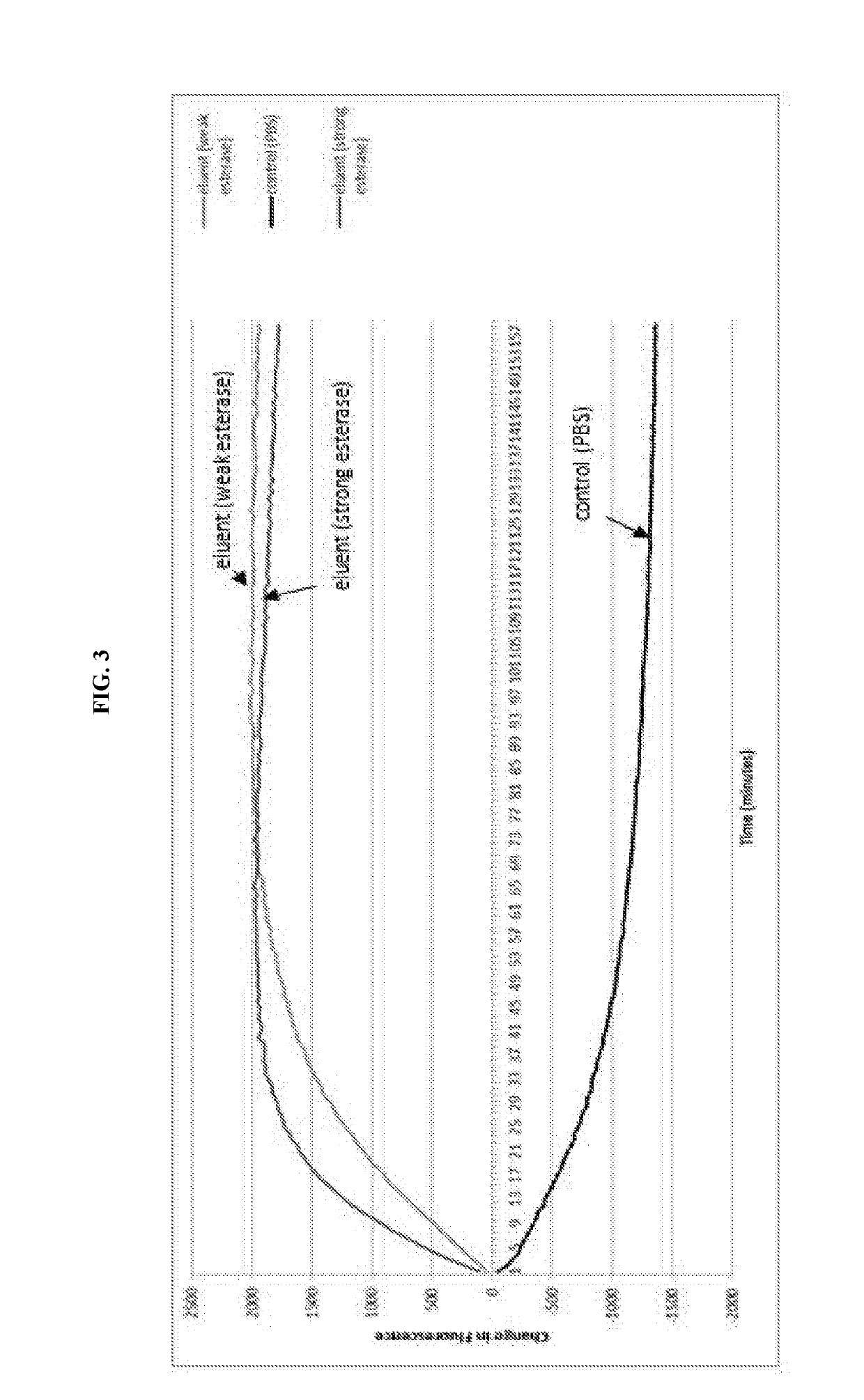
Modified wound dressings
a technology for wounds and dressings, applied in the field of wound healing, can solve the problems of reducing the quality of life, requiring a lot of care, and chronic and ‘infected’ wounds are typically difficult to heal, so as to improve the sensitivity, precision and specificity of infected wound detection, and improve the detection speed of infected wounds. the effect of exiting assays
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
on of Polymer 1 (Using CMC Fiber)
[0288]
[0289]Sodium carboxymethylcellulose (NaCMC) fiber (443 mg, 1.08 mmol) was dissolved in deionized water (44 ml) to give a 1% solution. Dowex 650C monosphere ion exchange resin was added in order to provide the acidified CMC (CMC-H). The monospheres were removed by filtration and then tetrabutylammonium hydroxide (TBAH) 40% (aq) was added until the pH was 8-9. The resulting solution was stirred for 30 min before being lyophilized overnight. The lyophilized material (0.38 g) was dissolved in 40 ml dry DMF under nitrogen with stirring and gentle heating over a period of approximately 1 h. The resulting solution was cloudy and off-white. The solution was cooled to approximately 4° C. To this stirred solution, 2-chloro-N-methylpyridinium iodide (CMP-I) (0.33 g, 1.3 mmol) was added. Shortly after this, compound 1-b (0.5 g, 2 mmol) was also added along with 3 drops of dry DCM and a few drops of dry triethylamine. The reaction mixture was kept at 4° C. ...
example 2
on of Polymer 2
[0294]
[0295]A solution of Fmoc-Phe-OH (0.0267 g, 0.069 mmol), HBTU (0.0262 g, 0.069 mmol), DIPEA (0.015 ml, 0.090 mmol) and dry DMF (0.82 ml) was prepared. A 15-ml plastic separating column was set up with a 10 m polyethylene frit and a luer tip was attached. Polymer 1 (0.034 g, 0.097 mmol) was added to the column followed by the amino acid solution. A lid was placed onto the column and was further sealed with parafilm. The column was placed on a blood rotor at RT overnight. The solution was drained using reduced pressure and the solid product was washed with EtOH to provide Polymer 2 as a white solid. Weight=0.0266 g. A Kaiser test was used to confirm absence of terminal amines. FTIR: 3324 (N—H / O—H), 3281 (O—H), 2898 (C—H), 1720 (aromatic C═O), 1666 (C═O urethane stretch), 1660, (C═O amide stretch of Fmoc).
[0296]Solubility of Polymer 2 was determined in a similar manner as for compound 1-c. Data is shown below: x indicates insoluble material.
AfterAfterSolventInitialh...
example 3
on of Polymer 3
[0297]
[0298]Polymer 2 was placed in a solution of piperidine: DMF (1:4 v / v, 6 ml) for approximately 2 h. The resulting de-protected product was then washed with EtOH before conducting a Kaiser test to confirm presence of free amine. This de-protected product (0.080 g, 0.15 mmol) was added to a 15-ml plastic separating column set up with a 10 μm polyethylene frit and a luer tip. A solution of Fmoc-Phe-OH (0.063 g, 0.16 mmol), HBTU (0.062 g, 0.16 mmol), DIPEA (0.23 ml, 1.8 mmol) and dry DMF (6 ml) was prepared and added to the column. A lid was placed onto the column and was further sealed with parafilm. The column was placed on a blood rotor at RT overnight. The solution was drained using reduced pressure and the solid product was washed with EtOH (10 ml×3) to provide the coupling product as a red solid. Weight=0.0691 g. A Kaiser test was used to confirm absence of terminal amines. The coupling product was washed with DCM. FTIR: 3315 (N—H / O—H), 2866 (C—H), 1730 (aromat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Acidity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com