Apparatus, systems and methods for transvascular access to the brain
a brain and transvascular technology, applied in the field of brain access apparatus, can solve the problems of increased hospital stay, neurologic deficit, and even death, and the conventional methods of accessing brain tissue are prone to surgery complications, and the hospital admissions are associated with high costs and significant complications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0065]The present disclosure describes a system in which a guide / access catheter provides an endovascular conduit for co-axial catheter systems and transcatheter instrumentation to transvascularly access the subdural space, subarachnoid space, and the brain parenchyma—for targeted device delivery or implantation and / or tissue / media insertion / collection without the needs for burr holes or craniotomy. Various novel neuroendovascular transvenous access / guide catheter designs and a variety of clinical applications that the catheter(s) would enable are described herein, such as for the diagnosis and treatment of seizure disorder, pathologic brain tissue (e.g., cancer, etc.), psychiatric disease—cognitive / motor impairment, and movement disorder.
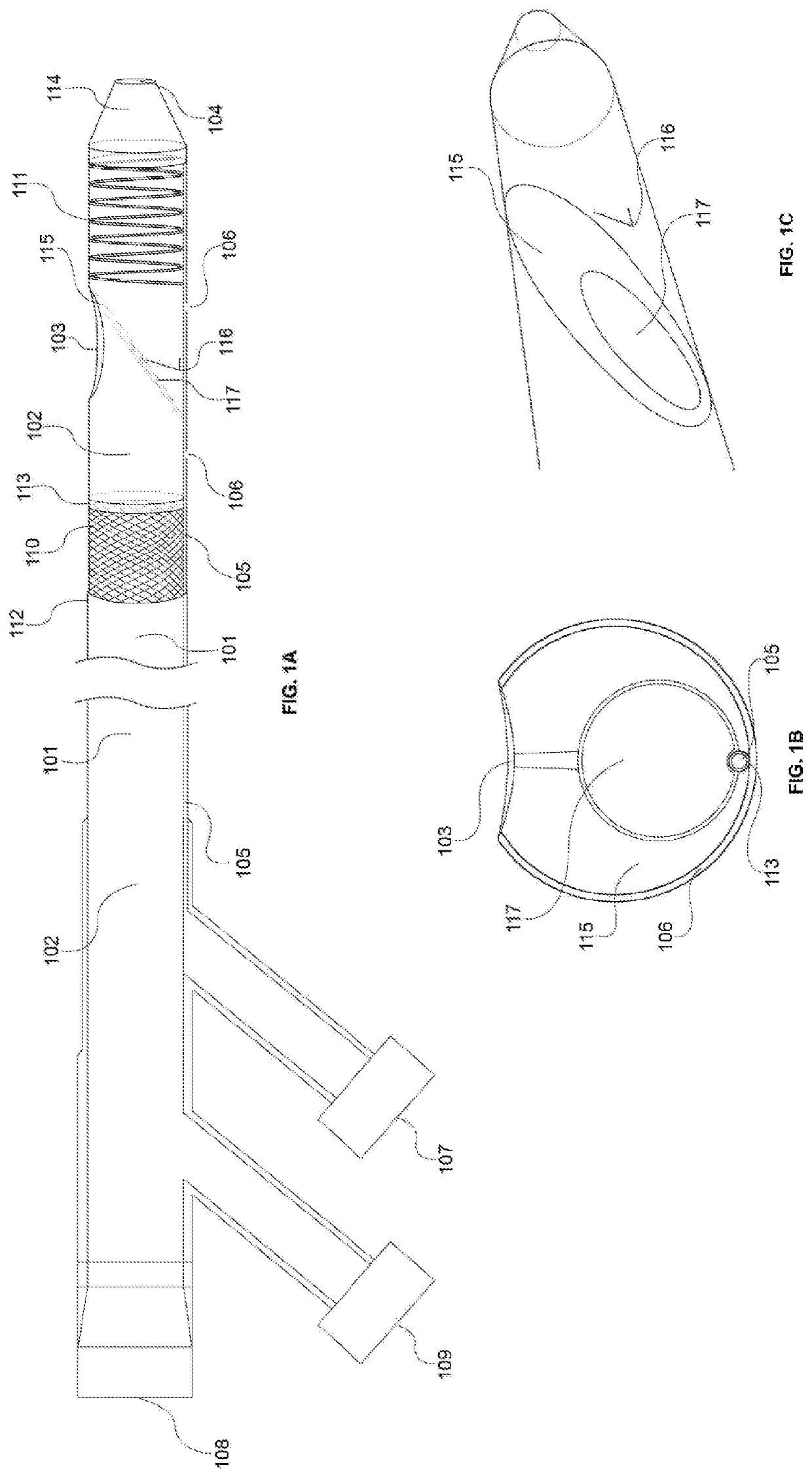
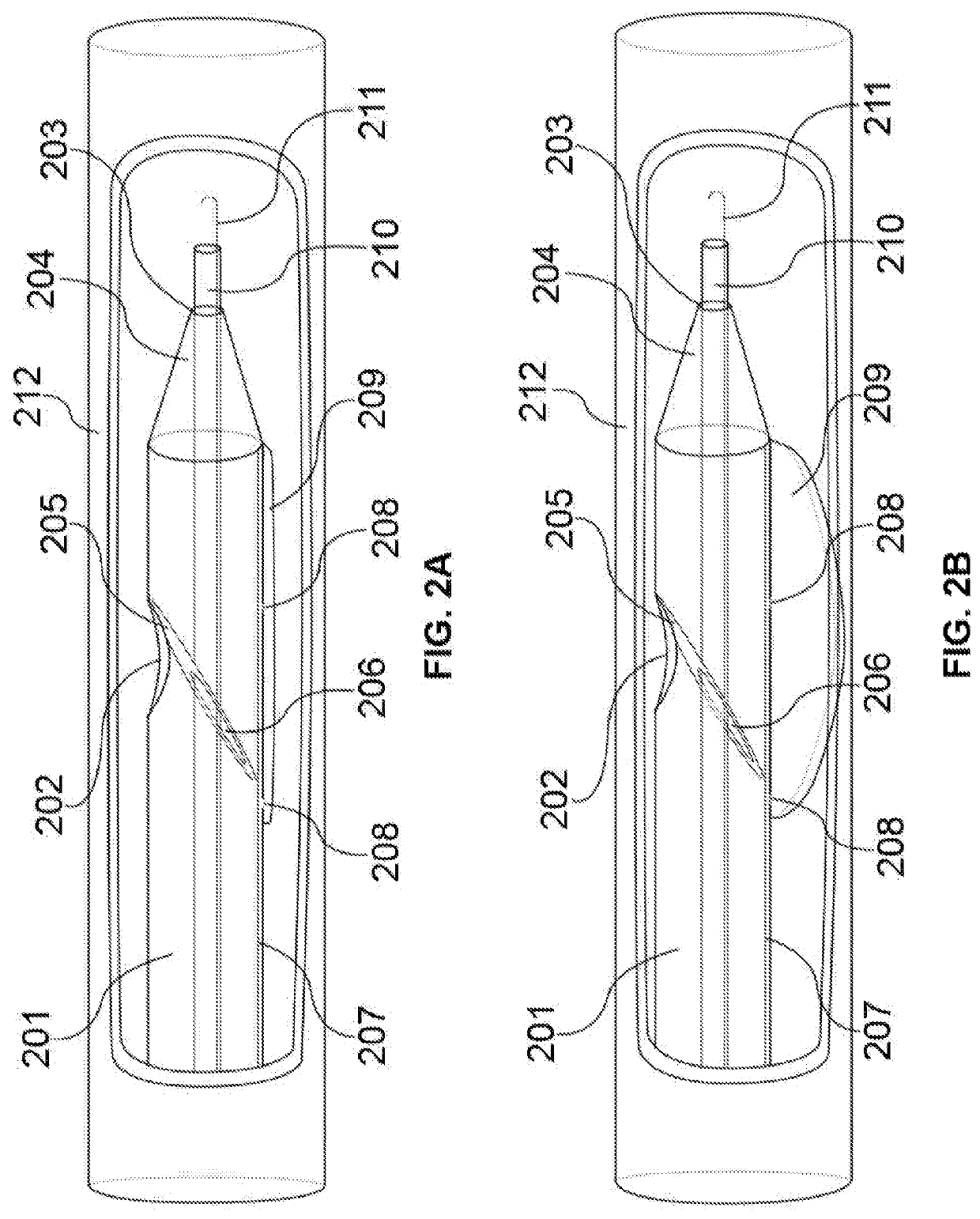
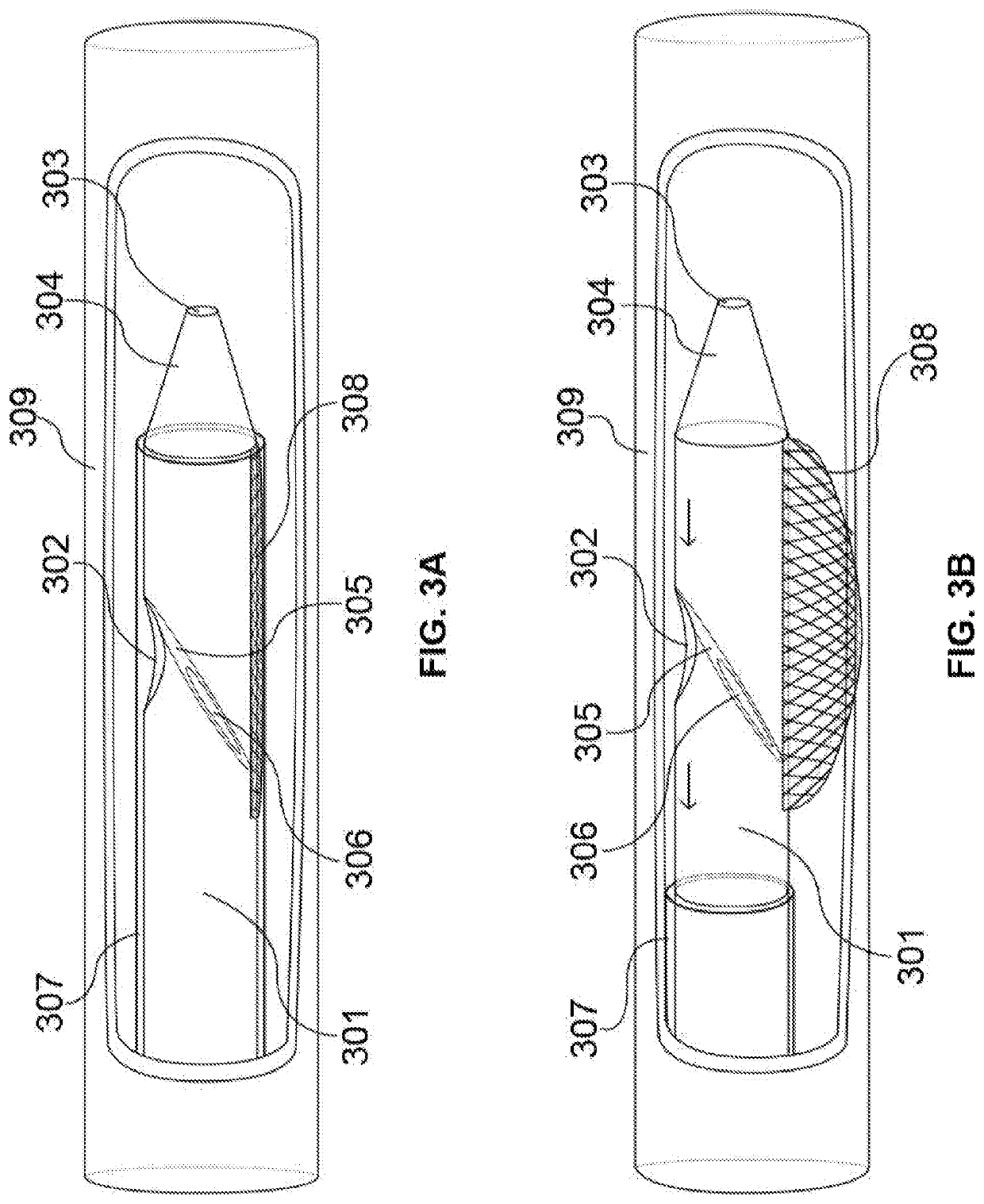
[0066]FIG. 1A-C illustrates a schematic diagram of the access catheter device comprising tubular structures featuring proximal and distal ends operably connected through lumen(s), cut-away views of various layers and inner components / features, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com