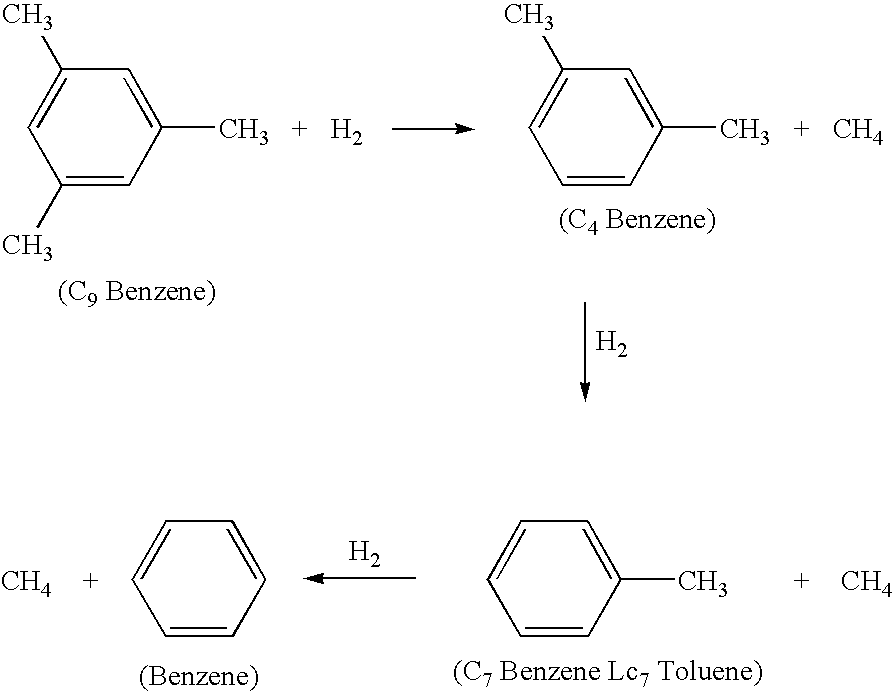
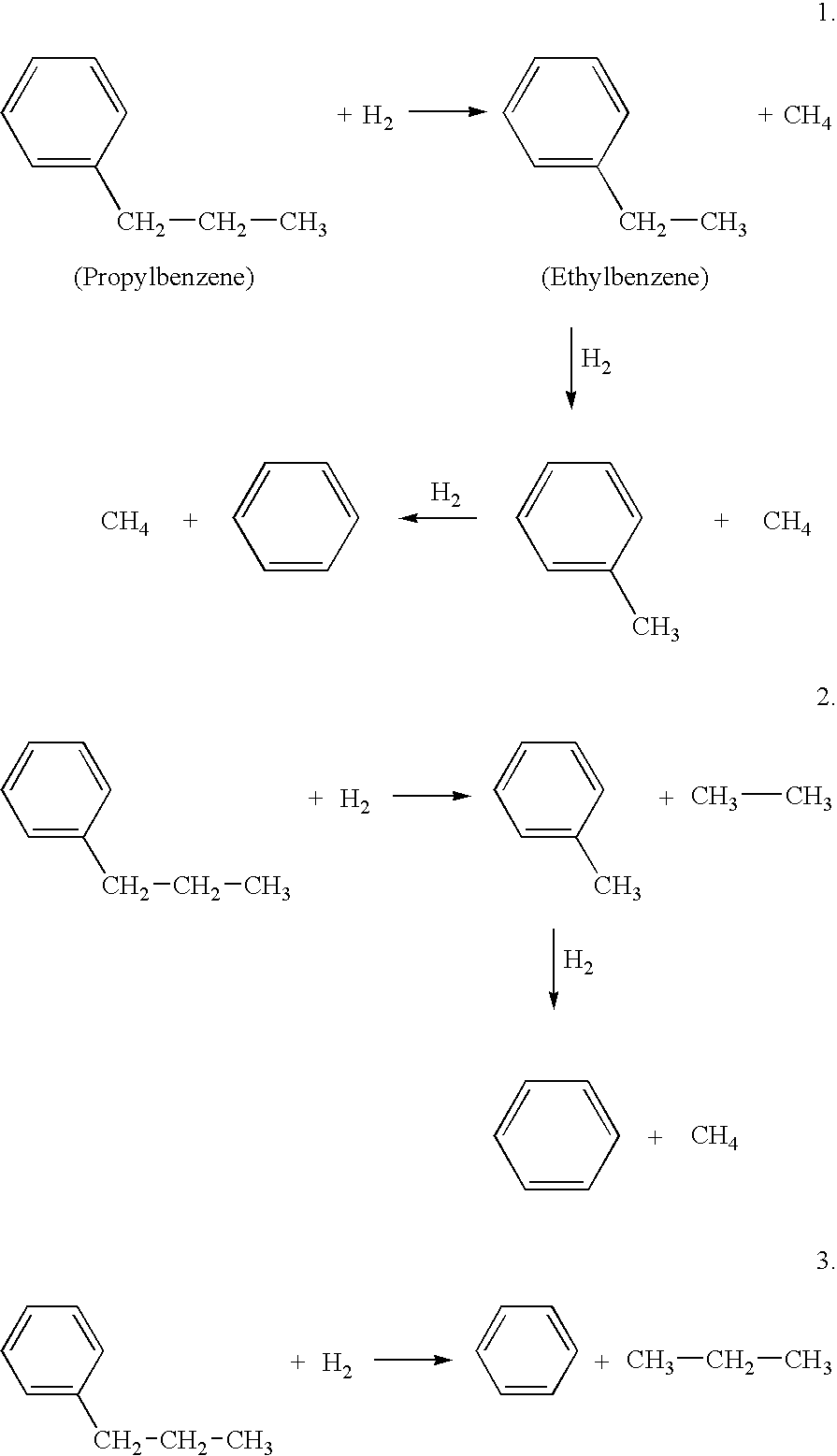
Hydrodealkylation processes
a technology of hydrodealkylation and process, applied in chemical/physical/physical-chemical processes, chemical/physical/physical-chemical processes, and preparation of oxygen-containing compounds, etc., can solve the problems of metal dusting, no longer existintiori protection, and sulfur interference in the carburization reaction, so as to reduce sulfide corrosion, improve product value, and increase sulfur levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
A thermal hydrodealkylation reactor made of 0.25 inch OD 316 stainless steel seamless pipe 8.75 inches long was prepared by cleaning the surfaces of the reactor exposed to the hydrodealkylation reaction with soap and water, and drying with an organic solvent. The reactor was then coated by pouring a tin paint into one end of the reactor, draining the excess, pouring the tin paint into the other end of the reactor, draining the excess, and then reducing the tin paint coating at approximately 1050.degree. F. for approximately 40 hours. The tin paint used for, coating the reactor was prepared by mixing together by weight 7 parts Tin Ten Cem (Mooney Chemical, Co.), 6 parts isopropyl alcohol, 14 parts tin powder (1-5 microns) and 14 parts stannic oxide (-325 mesh) and 5% Fe.sub.2 O.sub.3 in paint mixture. Toluene at 24 .mu.l / min was fed into the reactor with nitrogen at 20 cc / min for approximately 596 hours of operation at 1400.degree. F. No plugging occurred but operational problems in ...
example 2
A thermal hydrodealkylation reactor was prepared as described in Example 1 except that no Fe.sub.2 O.sub.3 was used in the paint. Into the reactor which was maintained at 1250.degree. F. and 100 psig, toluene at 25 .mu.l / min and hydrogen at 20 cc / mm were introduced for approximately 88 hours of operation. Then, the temperature was raised to 1400.degree. F. The reaction was continued until approximately 303 hours of operation occurred. No plugging of the reactor occurred.
example 3
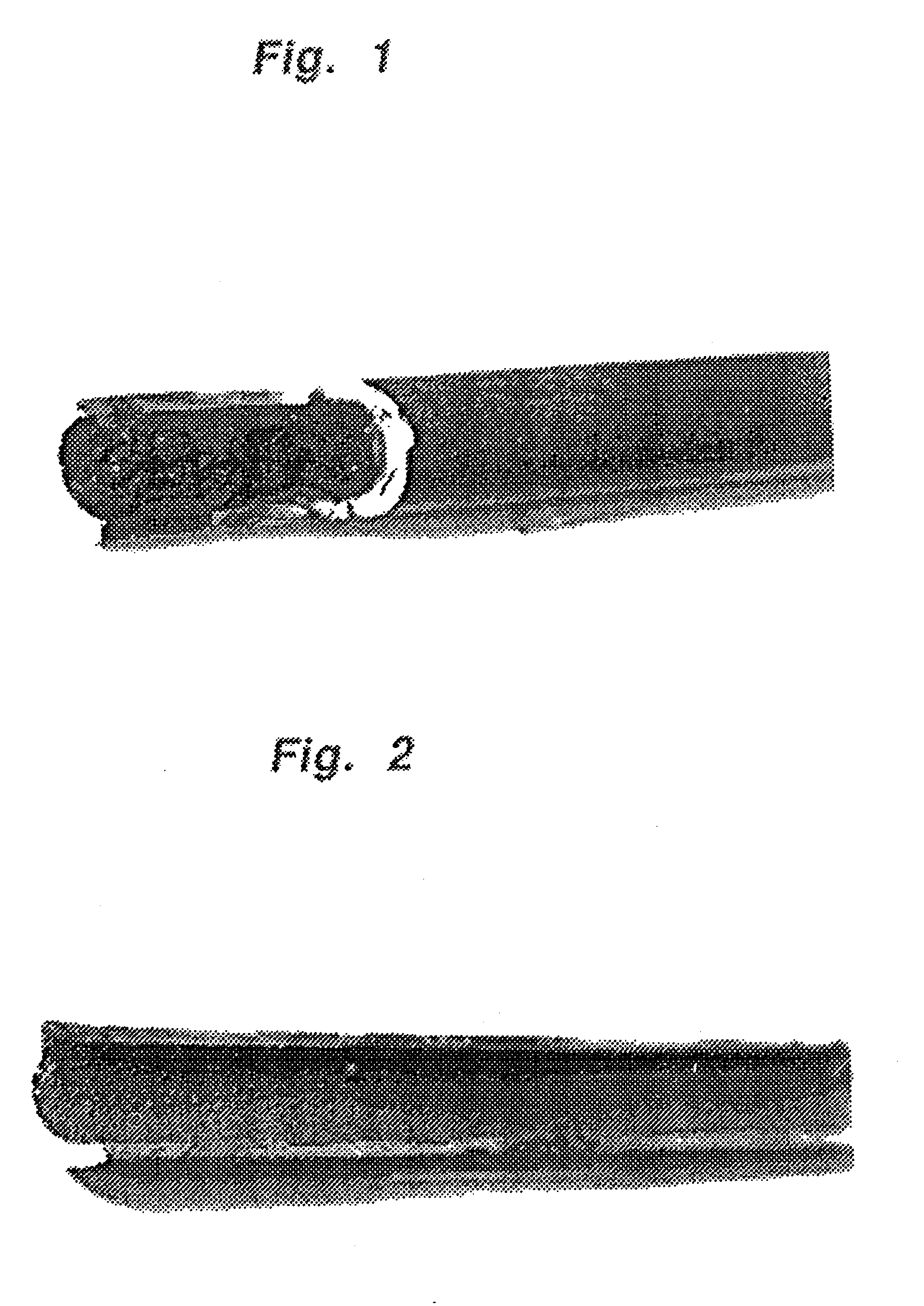
A thermal hydrodealkylation reactor was prepared as described in Example 1. The reactor was preheated and maintained at a temperature of 1400.degree. F. Toluene at 25 .mu.l / min was fed into the reactor with hydrogen at 10 cc / min. The reactor pressure was maintained at approximately 100 psig. The reaction was allowed to proceed for at least 597 hours before changing the feed to n-hexane. No plugging of the reactor occurred during approximately 600 hours of operation. See FIG. 2 which is a photograph of the reactor cut open to show that no plugging occurred.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com