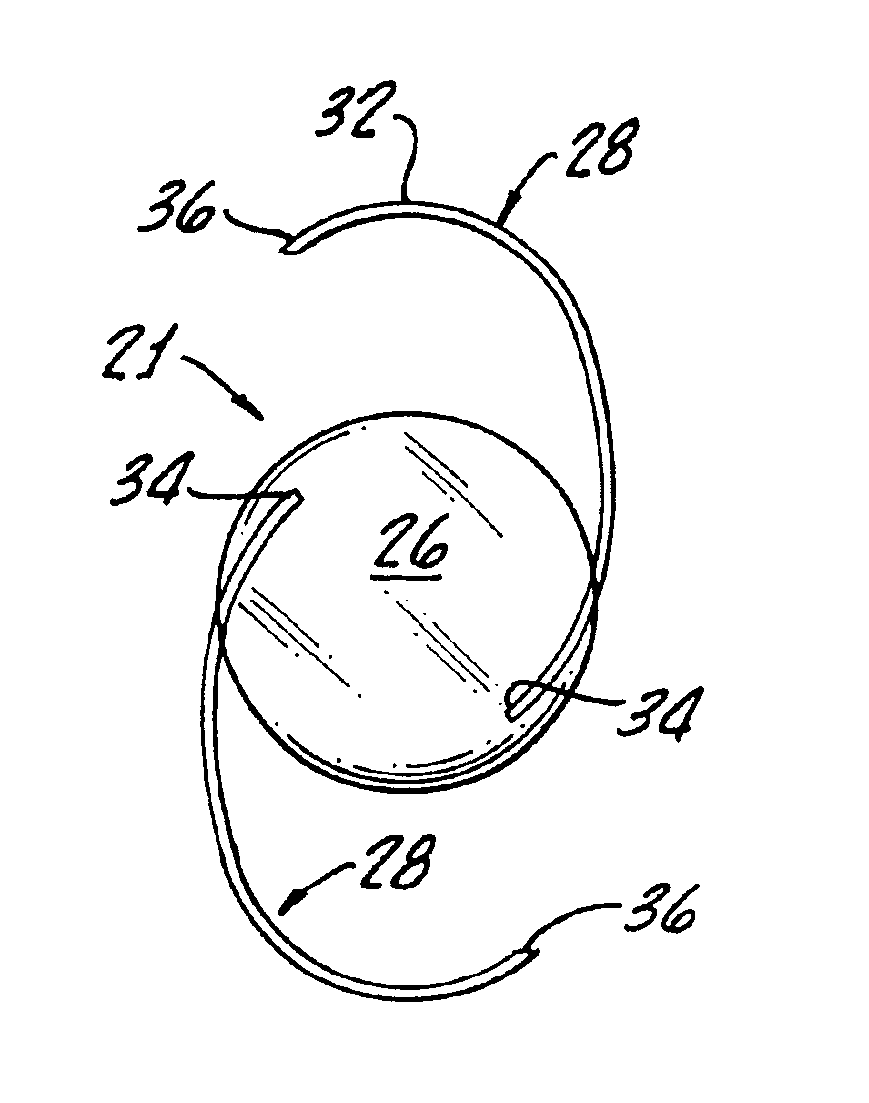
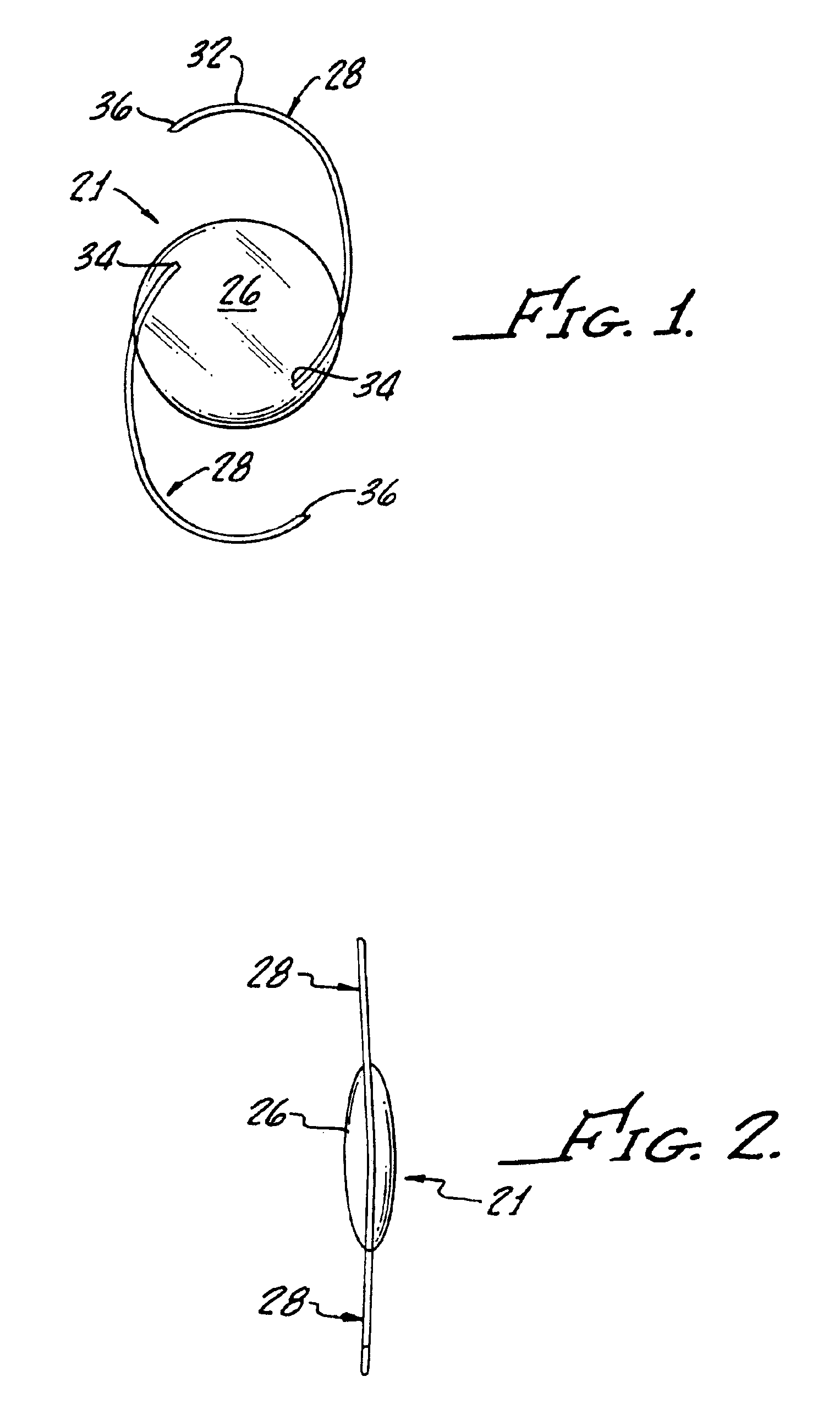
Intraocular lenses made from polymeric compositions and monomers useful in said compositions
a polymer composition and monomer technology, applied in the field of ophthalmic lenses, can solve the problems of deformation of acrylic materials that can be quite tacky, handling problems, disadvantageous decrease in the index of refraction of the final iol optic, etc., and achieve the effect of reducing surface tackiness, reducing surface tackiness, and reducing surface tackiness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0060]Synthesis of 2-phenylpropyl methacrylate
[0061]This monomer is prepared as follows:
[0062]A two-mouth one-liter round bottom flask containing a magnetic stir bar is lowered into an ice bath on top of a magnetic stirrer. 25 grams (0.18 mol) of 2-phenyl-1-propanol is added into the flask, followed by the addition of 30 ml (0.22 mol) of triethylamine to the flask. The now empty triethylamine addition flask is rinsed with about 10-ml of anhydrous diethyl ether and this is added to the round bottom flask. Another 200 ml of anhydrous diethyl ether is added to the round bottom flask, followed by 5 mg (0.0002 mol) of inhibitor (2,5-diphenyl-p-benzophenone). The magnetic stirrer stirs the resulting mixture at 555 rpm.
[0063]100 ml of anhydrous diethyl ether and 20-ml (0.21 mol) methacryloyl chloride are added, dropwise, while the contents of the flask are maintained at about 2° C. The methacryloyl chloride addition are adjusted so that the addition is completed in about 1½ hours. The reac...
example 2
Synthesis of 2-phenylpropyl acrylate
[0066]The above monomer is prepared as in Example 1 except that acryloyl chloride replaces methacryloyl chloride on an equal molar basis.
example 3
[0067]The following formulation is blended, purged with nitrogen for 3 minutes and then cured into a crosslinked copolymer.
[0068]
Quantity% Quantity2-phenylpropyl methacrylate15.0 g33.4%2-phenylpropyl acrylate21.5 g47.9%n-hexyl acrylate 6.5 g14.5%EGDMA ethylene glycol dimethacrylate 0.9 g 2.0%thermal initiator 0.1 g 0.2%(2,5-dimethyl-2,5bis(2-ethylhexanoylperoxy) hexaneUV absorber 0.9 g 2.0%
[0069]The resulting crosslinked copolymer has an index of refraction of 1.5396, hardness (Shore A) of 42, haze (after soaking) of 3, excellent optical transparency (clarity) and good mechanical properties, including low or reduced tackiness. A one cm diameter rod of this copolymer is folded 180° with no cracking and returns to its original shape within a few seconds.
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com