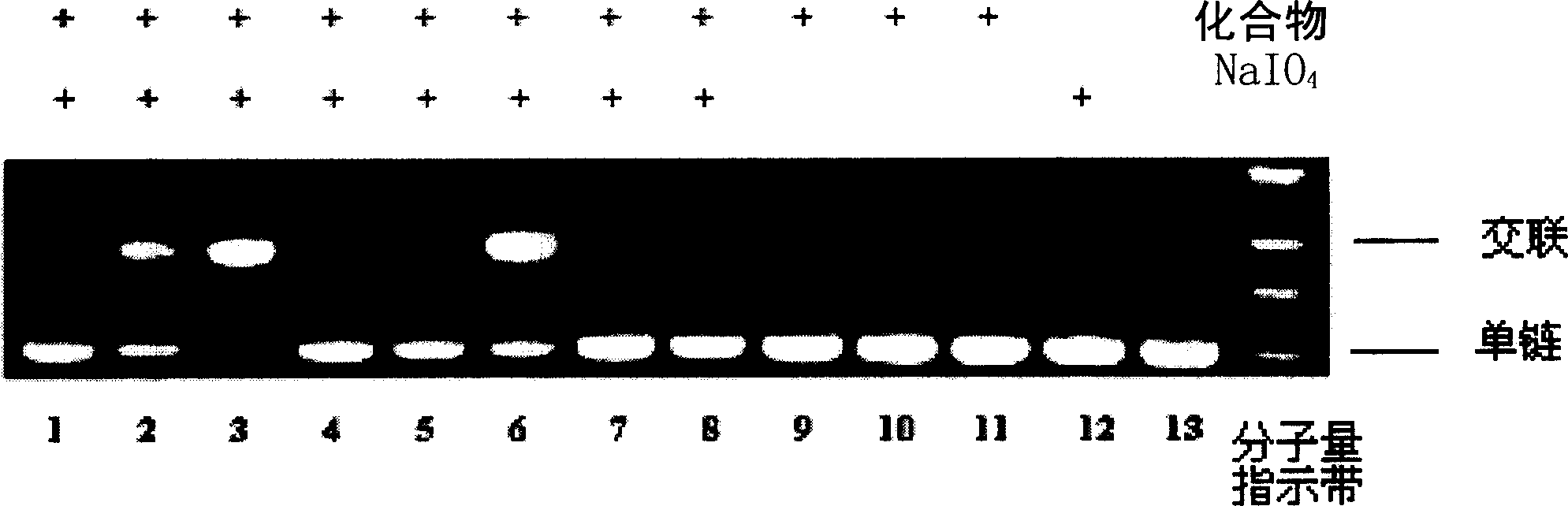Compound for generating nucleic acid cross-linking action by induction and its preparing method and use
A technology of cross-linking and compounds, which is applied in the direction of active ingredients of hydroxyl compounds, organic chemistry, drug combination, etc., can solve problems such as side effects and no specific recognition of tumor cells, and achieve the effect of simple preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
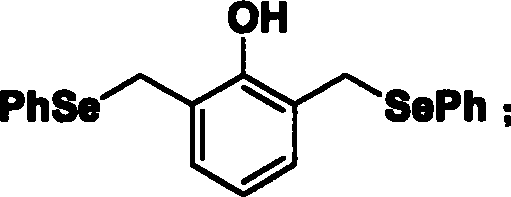
[0029] Synthesis of 2,6-bis(selenophenylmethylene)phenol (compound 1):
[0030]
[0031]Dissolve 452mg of 2,6-dimethylphenol in 10ml of N,N-dimethylformamide, add 1g of imidazole and 1.12g of tert-butyldimethylsilyl chloride (TBDMSCl) to react overnight at room temperature, and the reaction ends Afterwards, the solvent was evaporated and the product was separated by column chromatography (ethyl acetate:cyclohexane=1:120) to obtain 825 mg of compound 1a. Compound 1a was dissolved in carbon tetrachloride (CCl 4 ), adding 2.5 times the amount of N-bromosuccinimide (NBS), heated to reflux, then added 20 mg of azobisisobutylcyanide, refluxed for 2 hours, evaporated to dryness, and separated by column chromatography product (ethyl acetate:cyclohexane=1:120) to obtain 350 mg of compound 1b.
[0032] Under the protection of nitrogen, first diphenyl diselenide ((PhSe) 2 ) 474mg and sodium borohydride (NaBH 4 ) 114mg dissolved in anhydrous N, N-dimethylformamide (DMF), stirred at...
Embodiment 2
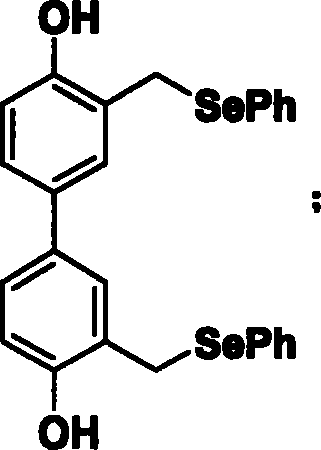
[0035] Synthesis of 4,4'-dihydroxy-3,3'-bis(selenophenylmethylene)biphenyl (compound 2):
[0036]
[0037] 1.86g biphenol was dissolved in 100ml acetone (acetone) and 10ml N,N-dimethylformamide, added 6.9 grams of anhydrous potassium carbonate solid, then added excess methyl iodide (CH 3 1) 5ml of the solution was stirred at room temperature for 8 hours, the reaction solution was extracted with chloroform and water, and the chloroform layer was evaporated to dryness to obtain 1.9g of white solid compound 2a. To 1.9 gram compound 2a and 1.332g paraformaldehyde (CH 2 (2) add 50ml acetic acid, heat to 80°C, then add 5ml 40% mass percent concentration of hydrobromic acid solution, react at 80°C for 3 hours, cool overnight at 4°C, filter the precipitated solid, and use dichloromethane dissolved, washed several times with saturated sodium bicarbonate solution, evaporated to dryness, and separated the product by column chromatography (ethyl acetate:cyclohexane=1:10) to obtain 920...
Embodiment 3
[0039] Synthesis of 1,4-dihydroxy-2,5-bis(selenophenylmethylene)phenol (compound 3):
[0040]
[0041] Under the condition of avoiding light, dissolve 1.1g hydroquinone in 100ml water, add 1.12g potassium hydroxide solid, then add 2.84ml dimethyl sulfate solution, stir at room temperature for 8 hours under nitrogen protection, a large amount of precipitate precipitates, filter and wash with water The solid was repeated several times to obtain 1.045 g of white solid compound 3a. Add 50ml of acetic acid to 1 gram of compound 3a and 1.087g of paraformaldehyde, heat to 60°C, then add 5ml of 40% by mass hydrobromic acid solution, react at 60°C for 4 hours, and cool overnight at 0°C , the precipitated solid was filtered, dissolved in dichloromethane, washed several times with saturated sodium bicarbonate solution, and evaporated to dryness to obtain 1.630 g of compound 3b. Under the protection of nitrogen, first dissolve 1.928 g of diphenyldiselenide and 458 mg of sodium borohyd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com