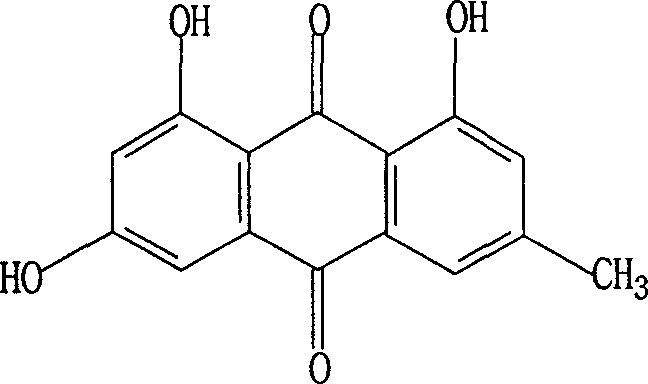
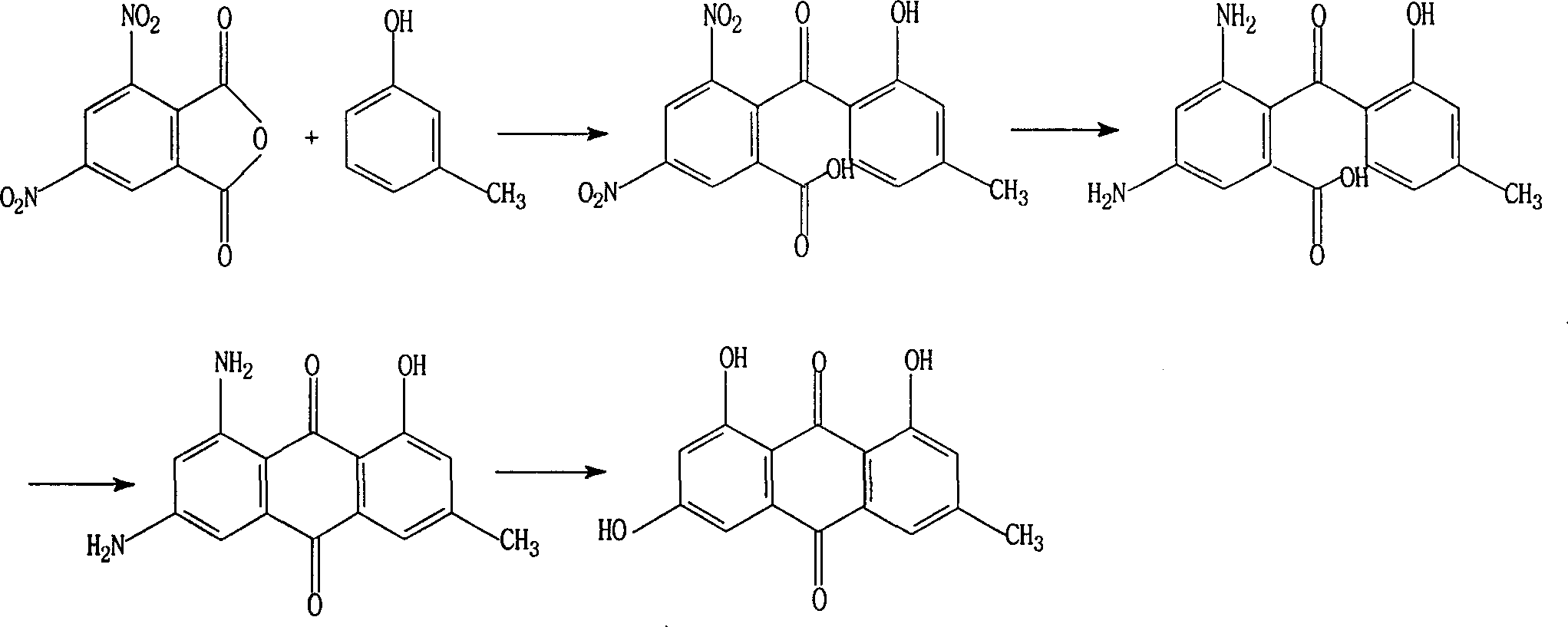
Improved method of synthesizing emodin
A technology of emodin and cyclization, which is applied in the field of synthesis and improvement of the main component emodin, can solve the problem of low yield and achieve the effect of increasing the total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1, the preparation of 2-(2-hydroxyl-4-methylbenzoyl)-3,5-dinitrobenzoic acid
[0022] Take by weighing 3,5-dinitrophthalic anhydride 119.0g (0.5mol) and m-cresol 400g (3.7mol), drop into and be equipped with sealed stirrer, anhydrous calcium chloride drying tube and reflux condenser In a 2L three-neck flask, cool the flask in an ice-water bath, add 200g (1.5mol) of powdered anhydrous aluminum trichloride at a time, start stirring, heat up, heat to 100-110°C, and stir vigorously for 3 hours until the release of hydrogen chloride stops , a viscous reddish-brown substance is produced. Ice was added while stirring until the excess chromium trichloride passed through the addition of 150ml of concentrated hydrochloric acid, the product coagulated and the solution was clarified. After filtration, the filter cake was treated with 50 g of sodium carbonate, filtered, and dried to obtain 154.8 g of white crystal 2-(2-hydroxyl-4-methylbenzoyl)-3,5-dinitrobenzoic acid, y...
Embodiment 2
[0023] Embodiment two, the preparation of 2-(2-hydroxyl-4-methylbenzoyl)-3,5-diaminobenzoic acid
[0024] Put 346g (1mol) of 2-(2-hydroxy-4-methylbenzoyl)-3,5-dinitrobenzoic acid, 300ml of absolute ethanol and 5g of Raney nickel into a 1L autoclave. Close the autoclave, feed hydrogen, raise the temperature to 25°C to make the pressure 3.5Mpa, stir at a medium speed, rapidly raise the temperature to 90°C, stop heating, and maintain the reaction at 90-100°C for 8 hours. Cool, depressurize, and filter the catalyst. Ethanol was distilled off, and the residue was recrystallized to obtain 269 g of amino compounds, with a yield of 94.1%, mp: 232-234°C, and content (HPLC) ≥ 96.5%.
Embodiment 3
[0025] Example 3, Preparation of 1-hydroxyl-6,8-diamino-3-methylanthraquinone
[0026] Add 2-(2-hydroxyl-4-methylbenzoyl)-3,5-diaminobenzoic acid 28.6g (0.1mol) in the 500ml three-necked bottle, oleum 136ml, boric acid 65g, slowly heat up to React at 120°C for 1 hour, then raise the temperature to 150-160°C to make the solution transparent, neutralize with 10% sodium hydroxide solution, extract with ethyl acetate, evaporate to dryness, and then recrystallize with acetone and water to obtain 21.2 g of dark orange crystals , yield 79.1%, mp: 300-307°C, content (HPLC) ≥ 96.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com