Lappaconitine hydrobromide transparent patch and the preparing method thereof
A kind of technology of homogenin hydrobromide and transdermal patch, which is applied in the field of pharmacy, can solve the problems of high quality injection, high production cost, and inconvenient use, so as to improve the curative effect of the drug, reduce individual differences, and meet the needs of treatment. desired effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
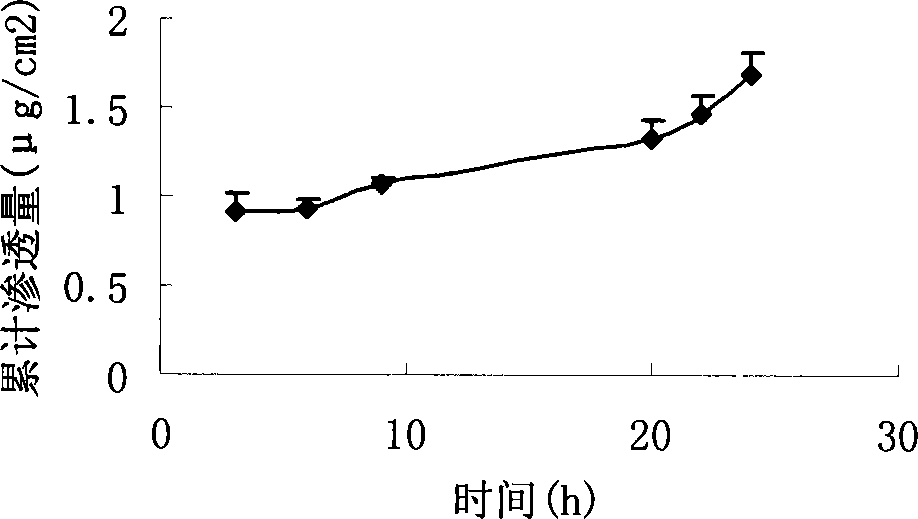
[0041] Weigh 29.4mg of genivirine hydrobromide, add 3ml of ethanol, and after ultrasonic dissolution, add 525mg of acrylic resin, ultrasonically dissolve and mix well. Coated on 42cm by salivation process 2 On the backing layer, evaporate the solvent naturally, dry and solidify in an oven at 80°C, and cover with a protective layer after cooling. A modified Franz diffusion cell device was used to measure its permeability to human skin in vitro, and the receiving solution was 20% polyethylene glycol 400 (PEG400) physiological saline aqueous solution. Use HPLC to measure uricine hydrobromide, using Agilent ZORBAX Eclipse×DB-C18 chromatographic column (4.6mm×150mm, 5μm), the column temperature is 35°C, and the mobile phase is 0.1mol.L -1 Sodium dihydrogen phosphate: methanol (1:1), detection wavelength 252nm, flow rate 1.0ml / min. The permeation rate is 0.09μg / cm 2 h. figure 1 It is the drug cumulative penetration-time curve (time is hours) of this embodiment.
Embodiment 2
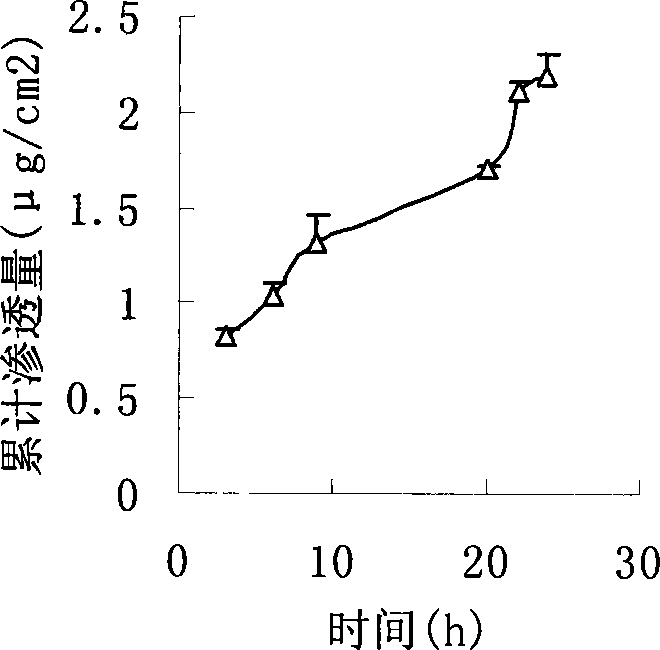
[0043] Weighed 29.4mg of urine hydrobromide, added 3ml of ethanol, ultrasonically dissolved, then added 525mg of acrylic resin, and then added 5.6mg of propylene glycol for ultrasonically dissolved and mixed. Coated on 42cm by salivation process 2 On the backing layer, evaporate the solvent naturally, dry and solidify in an oven at 80°C, and cover with a protective layer after cooling. The following operations are the same as Example 1. The permeation rate is 0.13μg / cm 2 h. figure 2 It is the drug cumulative penetration-time curve (time is hours) of this embodiment.
Embodiment 3
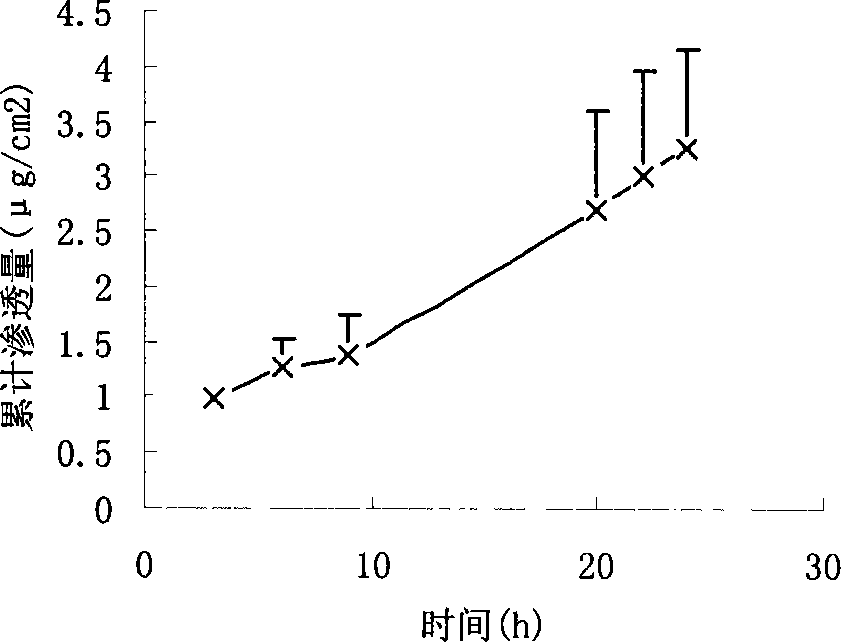
[0045] Weighed 29.4 mg of urine hydrobromide, added 3 ml of ethanol, ultrasonically dissolved, then added 525 mg of acrylic resin, and then added 17.1 mg of propylene glycol for ultrasonically dissolved and mixed. Coated on 42cm by salivation process 2 On the backing layer, evaporate the solvent naturally, dry and solidify in an oven at 80°C, and cover with a protective layer after cooling. The following operations are the same as in Example 1. The permeation rate is 0.12μg / cm 2 h. image 3 It is the drug cumulative penetration-time curve (time is hours) of this embodiment.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com