Low-temperature alkaline phosphatidase A1 and coding gene thereof
A phospholipase and alkaline technology, applied in genetic engineering, plant genetic improvement, hydrolytic enzymes, etc., to achieve the effect of simplifying the production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
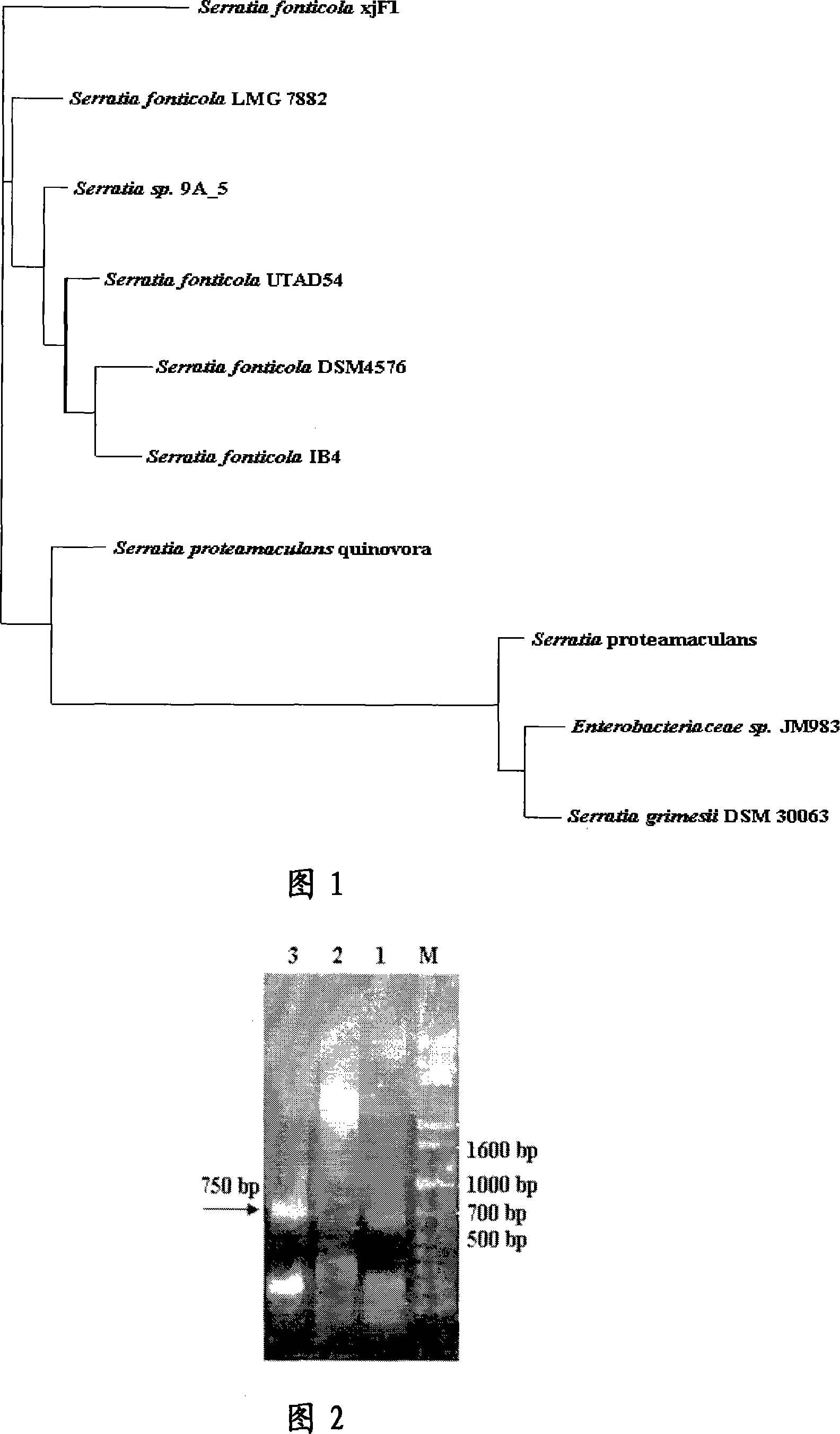
[0047] Embodiment 1: low temperature alkaline phospholipase A 1 Screening of producing bacteria
[0048] 1. Isolation and screening of strains
[0049] Collect Xinjiang Tianshan No. 1 glacier and permafrost, and apply it with 0.1% (NH 4 ) 2 SO 4 , 0.1%K 2 HPO 4 , 1% KCl, 0.05% MgSO 4 ·7H 2 O, 0.001% FeSO 4 ·7H 2 O, olive oil and polyvinyl alcohol emulsion mixed in a ratio of 1: 3, on the fat assimilation medium of 2% agar powder, cultivated at 20°C for 24 hours and screened 3 lipase-producing bacterial strains. Bacterial colonies were selected and spread on egg yolk+TYSPN solid agar medium (4% glucose, 1% peptone, 5% yeast extract, sterile egg yolk liquid, 2% agar powder, pH7.0). The strain xjF1 formed a yellow discoloration circle on the oil assimilation plate, and formed an opaque and cloudy halo on the egg yolk + TYSPN solid agar plate. Insert strain xjF1 into phospholipase production medium (5.4% peptone, 2.6% yeast extract, 1.512% NaH 2 PO 4 12H 2 O, 0.3%K ...
Embodiment 2
[0089] Example 2: Activity assay of enzymes produced by S. fonticola xjF1
[0090] low temperature alkaline phospholipase A 1 The activity was determined by KOH potentiometric titration (ie, modified Kawauchi method). The preparation of the substrate lecithin also refers to the Kawauchi method (Biochimica et Biophysica acta, 142-159, 1971). Lecithin 500mg, dissolved in 10mL diethyl ether, add 20mL double distilled water and mix well, bathe in 65℃ water for 25min to remove diethyl ether, then add 20mL double distilled water, emulsify in an ultrasonic generator for 40min in ice bath, add 1mol / L NaCl 10mL, 0.01mol / L CaCl 2 10mL, 0.01mol / L sodium deoxycholate 10mL, stir to dissolve, then dilute to 100mL with distilled water, finally adjust the pH value to 8.2-8.5 with 1.0mol / L NaOH, store at 4°C for no more than 48h. Take 10mL of substrate solution for each measurement, 200uL of enzyme solution to be tested, react at 37°C for 30min, add 6mL of 95% ethanol to terminate the reac...
Embodiment 3
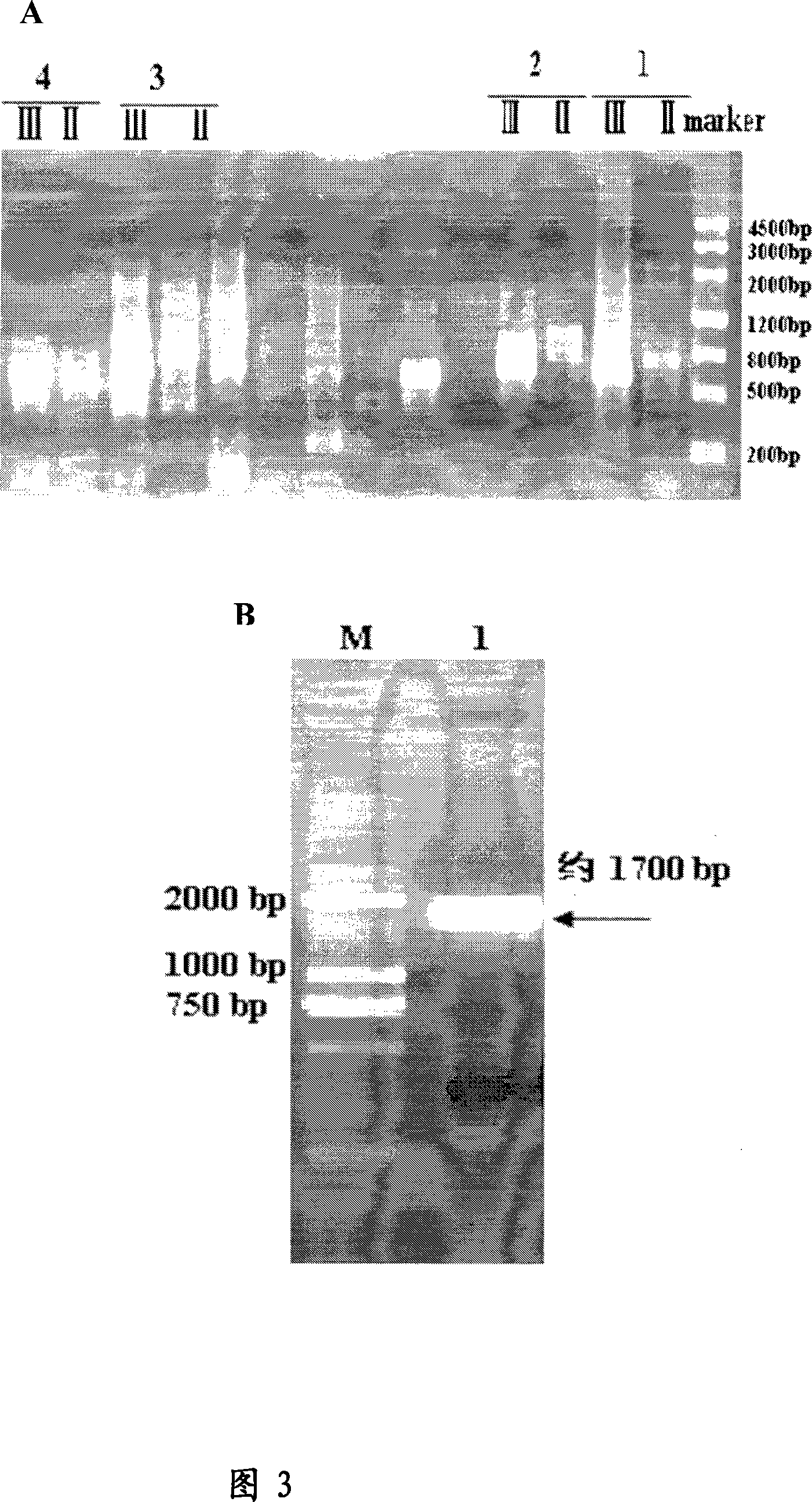
[0101] Example 3: Phospholipase A of S. fonticola xjF1 1 gene cloning
[0102] 1. Extraction of Genomic DNA from Serratia genus xjF1
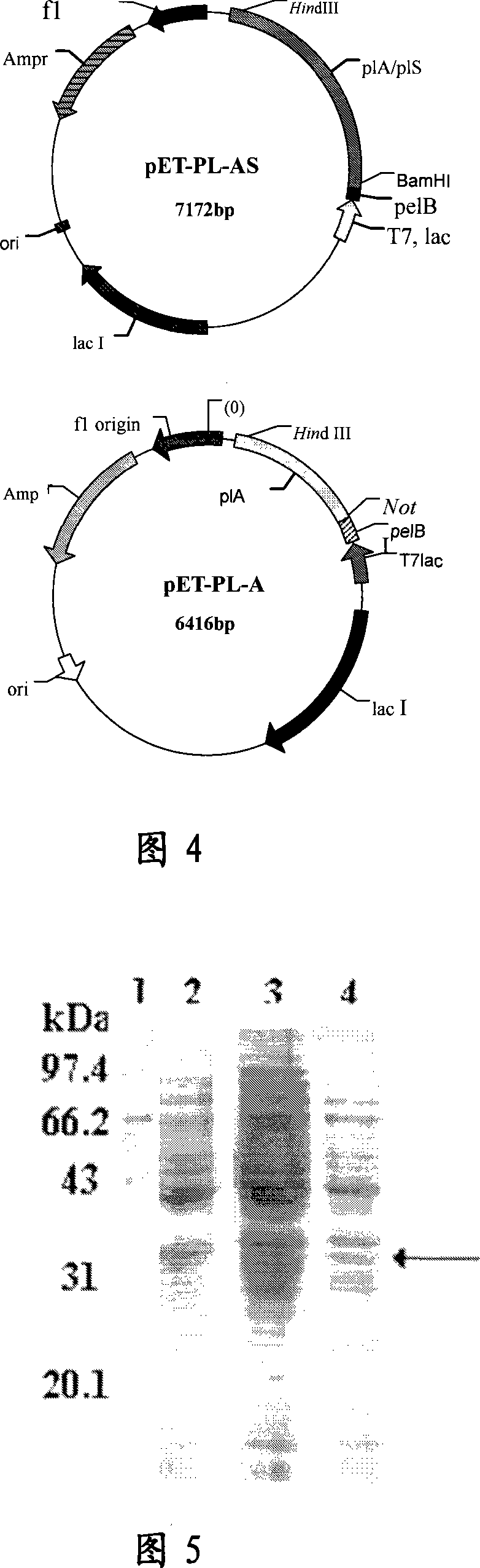
[0103] Genomic DNA of Serratia citiensis xjF1 was prepared by conventional methods known to those skilled in the art. Serratia genus xjF1 was cultured with shaking in LB liquid medium for 16 hours, and the supernatant was discarded by centrifugation. Take 50mg of bacteria sludge and add 500uL sterile water to wash. The pellet after centrifugation was resuspended in lysozyme mixture, incubated at 37°C for 30min, and then 10% SDS was added to a final concentration of 2%. The supernatant after centrifugation at 12000 rpm for 5 min was extracted with equal volumes of phenol, phenol:chloroform and chloroform in sequence. Take the upper layer solution and add isopropanol, precipitate at room temperature for 20 minutes, centrifuge at 12,000 rpm for 20 minutes, discard the supernatant, dissolve the DNA precipitate with sterile water, and store it a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com