Aldoforwe ester self-emulsifying capsule preparation and its making method
Adefovir dipivoxil, self-emulsification technology, applied in the direction of capsule delivery, digestive system, antiviral agent, etc., can solve the stability problem of adefovir dipivoxil, poor stability of active ingredients and easy degradation, workers' respiratory tract skin Diseases and other problems, to achieve the effect of beneficial gastrointestinal absorption, large self-emulsification, and easy quality control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
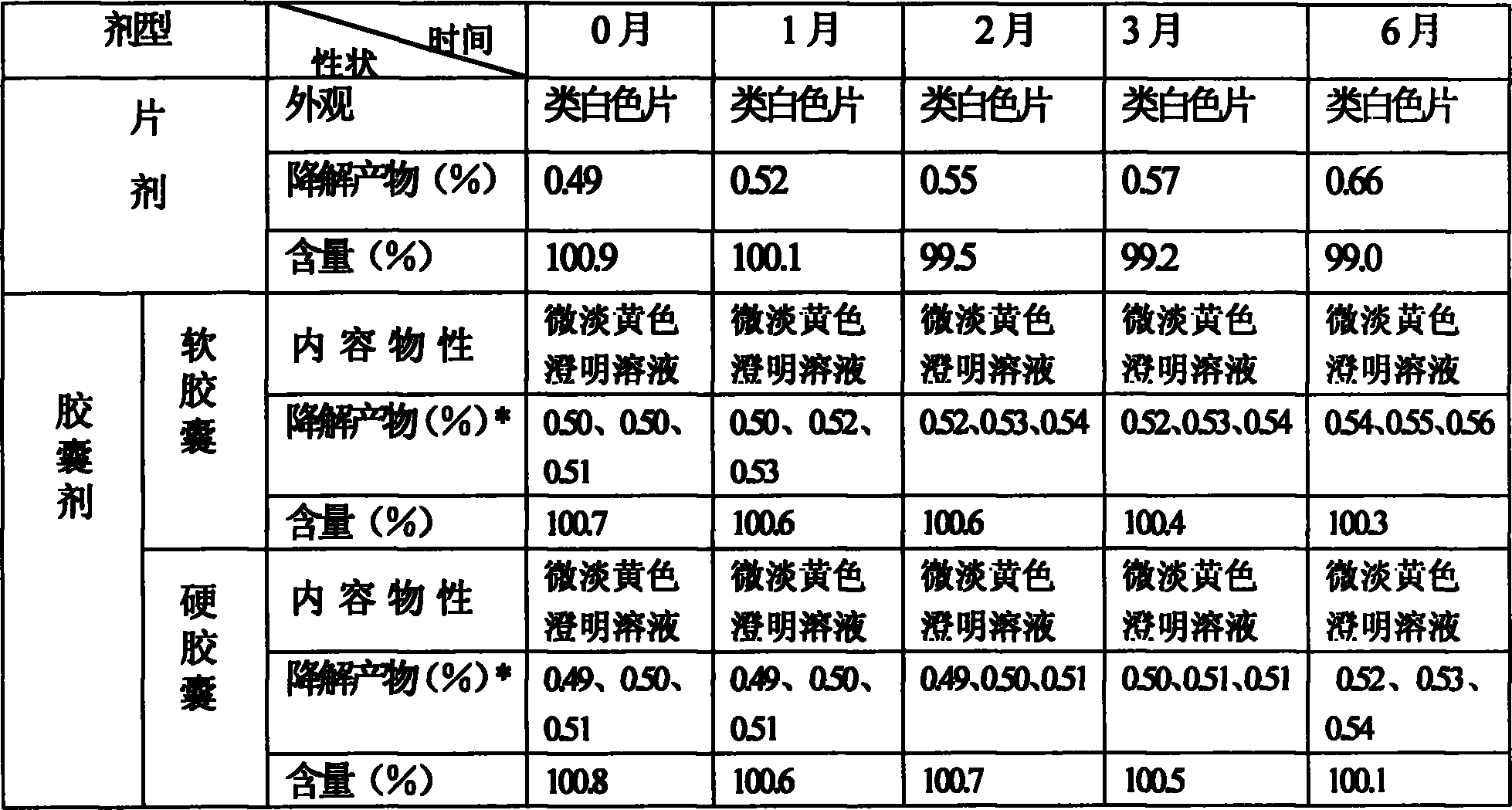
[0046] Take the ethyl acetate production intermediate solution containing 96g of adefovir dipivoxil and 1652g of caprylic diglyceride, mix well, and at a temperature of 50°C, completely volatilize the ethyl acetate by rotary thin film evaporation, and then add polyoxyethylene (40) 492 g of castor oil was heated in a water bath at 80° C. to a homogeneous and transparent solution, which was prepared into soft capsules with a specification of 12 mg of adefovir dipivoxil per unit by the compression method and an automatic rotary encapsulation machine.
[0047] The average particle diameter of the self-emulsifying capsule preparation after the in vitro emulsification test is 162nm, and the distribution range is between 150nm and 180nm, as determined by a Zetasizer Nano S-type nanoparticle size analyzer.
Embodiment 2
[0049] Get the tetrahydrofuran production intermediate solution containing 80g of adefovir dipivoxil and 2052g of caprylic triglycerides, mix well, and at a temperature of 50°C, use the rotary thin film evaporation method to completely volatilize the tetrahydrofuran, then add polyoxyethylene (60) to hydrogenate Castor oil 320g, water-bath ultrasonic to homogeneous, transparent solution, adopts compression method, is prepared into the soft capsule that per unit contains adefovir dipivoxil and is 10mg with automatic rotary encapsulation machine.
[0050] The average particle diameter of the self-emulsifying capsule preparation after the in vitro emulsification test is 223nm, and the distribution range is between 210nm and 260nm, as determined by a Zetasizer Nano S-type nanoparticle size analyzer.
Embodiment 3
[0052] Take the dichloromethane production intermediate solution containing 48g of adefovir dipivoxil and 2485g of capric acid monoglyceride, mix well, and at a temperature of 60°C, completely volatilize the dichloromethane with the rotary thin film evaporation method, then add polysorbate Alcohol ester 85 is 427g, water bath is ultrasonicated to homogeneous, transparent solution, adopts compression method, is prepared into the soft capsule that per unit contains adefovir dipivoxil with the specification of 6mg with automatic rotary encapsulation machine.
[0053] The average particle diameter of the self-emulsifying capsule preparation after the in vitro emulsification test is 230nm, and the distribution range is between 180nm and 250nm, as determined by a Zetasizer Nano S-type nanoparticle size analyzer.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com