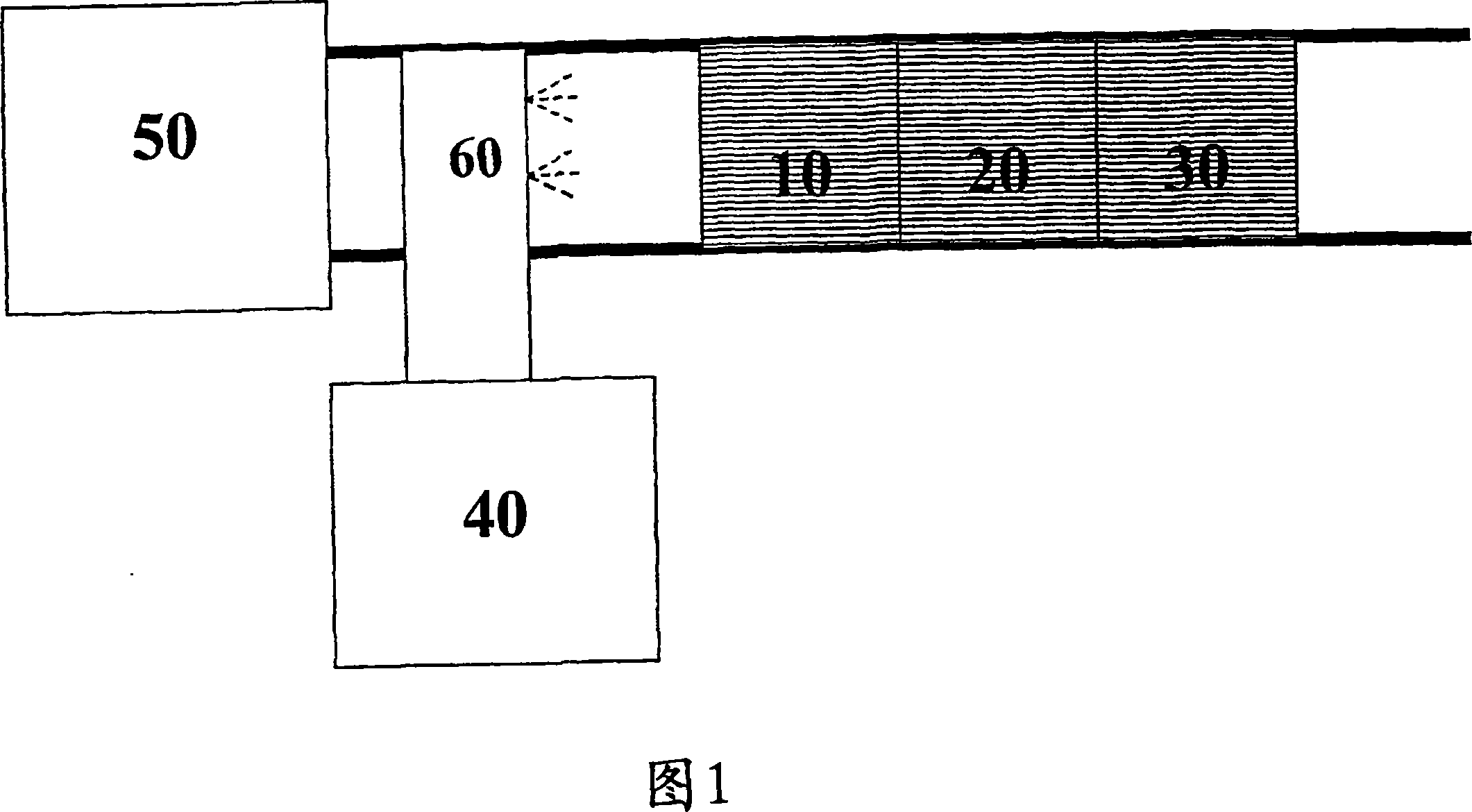
Catalyst and method for reducing nitrogen oxides in exhaust streams with hydrocarbons or alcohols
一种氮氧化物、催化剂的技术,应用在氮氧化物的催化剂领域,能够解决不适合空间有限的应用、不存在还原剂、不可重现性等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
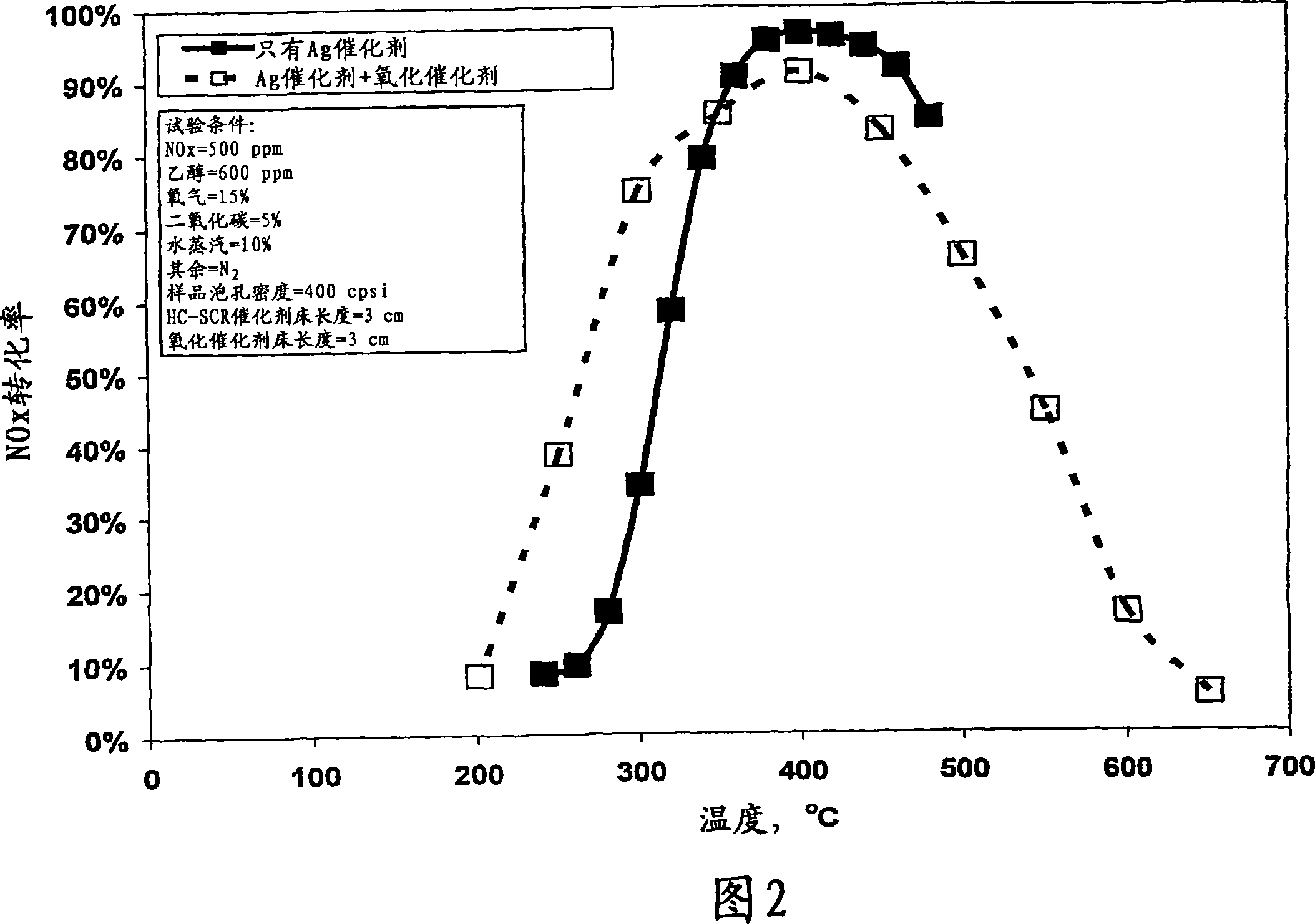
[0182] NO on silver on alumina catalyst with and without oxidation catalyst as a function of temperature x conversion rate of
[0183] Silver / alumina monolithic catalysts were prepared as described below. A slurry is formed by mixing water, silver nitrate and alumina. Ball mill this slurry, and at 400 cells / inch 2 Coating on a monolithic substrate of , and firing at 600 °C, yielded 1.2% silver on alumina monolithic substrate coated catalyst 10.
[0184] Figure 2 shows the results for 1.2% silver / Al with and without platinum on an alumina oxidation catalyst 30 placed in the feed stream after the silver catalyst as shown in Figure 1 2 o 3 Catalyst 10, NO x A graph of conversion versus temperature (°C). Contains 500ppmNO x Tests were conducted with a feed stream of 600 ppm ethanol. The feed gas also contains 15% oxygen, 5% carbon dioxide, 10% water vapor and the balance nitrogen. The flow rate of the raw material stream is maintained at 1 standard liter / minute, which cor...
Embodiment 2
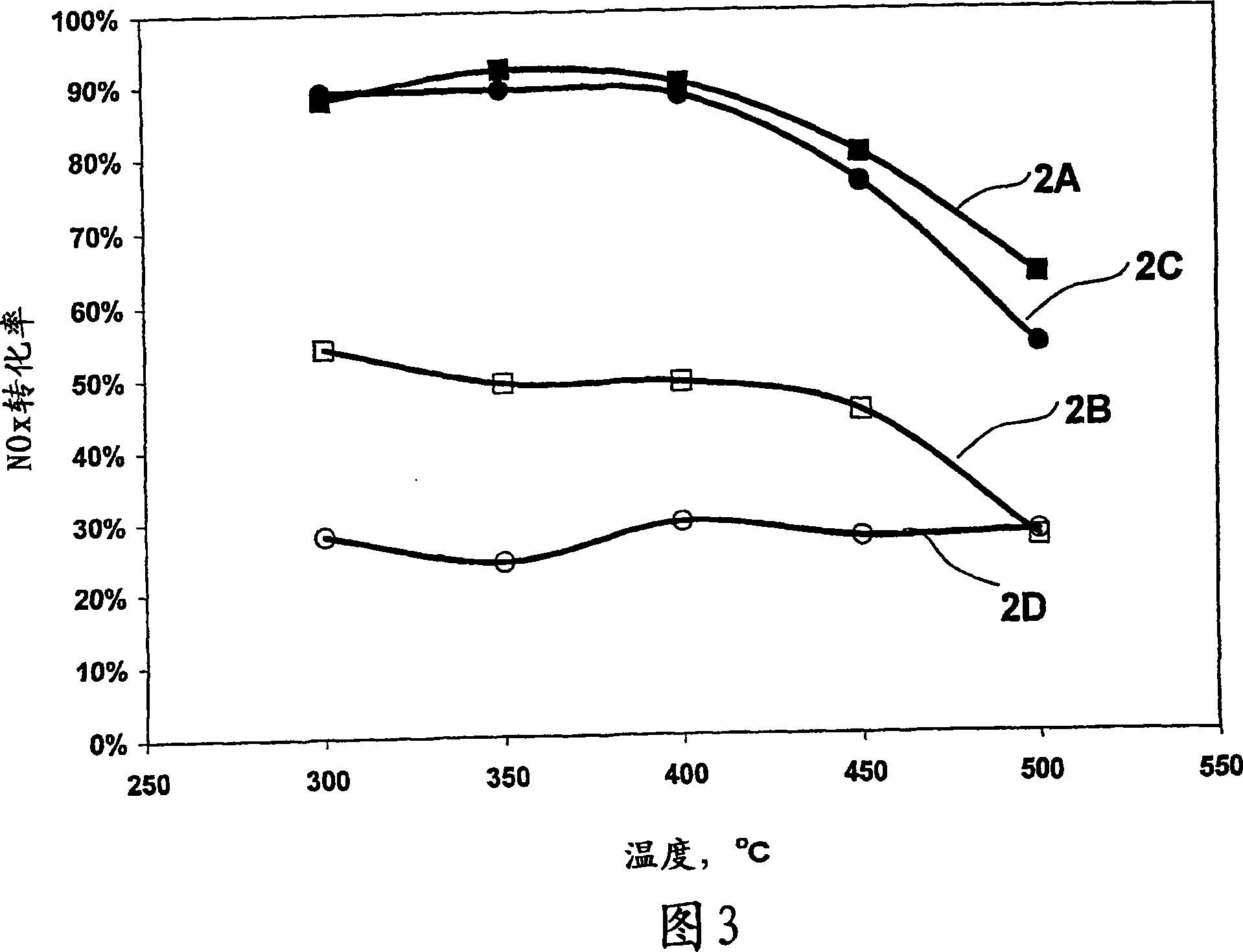
[0189] Contains 25ppmNO x In the case of a feed stream, with and without an oxidation catalyst, NO over a silver catalyst x Conversion rates
[0190] Silver / alumina monolithic catalysts were prepared using two different preparation methods. Catalyst 1 was prepared using the one-step carrier coating method described in Example 1.
[0191] Catalyst 2 was prepared in a two-step process by coating a substrate with an alumina support and then impregnating the alumina support coat with an aqueous solution of silver nitrate. Catalyst 2 was prepared as described below. An alumina slurry is formed by mixing alumina and water. Ball mill the slurry at 400 cells / inch 2 coated on monolithic substrates and dried. Silver nitrate was impregnated on the alumina coated monolith and fired at 600°C to yield 1.2% silver on alumina catalyst.
[0192] Table 2 shows the metal loading on the calcined monolith catalysts, where the percentage loading is calculated on a metal basis relative to the...
Embodiment 3
[0205] With and without oxidation catalyst, using propane as reductant, with 9ppm NO x feed stream, NO over silver / alumina catalyst x Conversion and CO production
[0206] Figure 4 shows the use of 9ppm NO x raw material gas, using 6000ppm propane as reducing agent, for silver / alumina catalyst, NO x A series of plots of conversion (left-hand axis) and outlet CO (right-hand axis) versus temperature for . The feed gas also contains 12% oxygen, 4% carbon dioxide and 10% water vapor, with the balance being nitrogen. at 8000h -1 Experiments were performed at a space velocity of .
[0207] When propane is used as reducing agent, as NO x A by-product of the conversion produces CO. CO is oxidized with an oxidation catalyst, thereby removing CO. However, in the presence of an oxidation catalyst, the apparent NO x The conversion rate dropped significantly, as shown in Figure 4. Oxidation catalysts are thought to oxidize the nitrogen-containing intermediates formed via Reaction...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com