Fixedpoint mutation modified phytase
A site-directed mutation and phytase technology, applied in the field of phytase, can solve the problems of no use value, waste of cost, loss of enzyme activity, etc., and achieve the effect of promoting large-scale industrialization and improving heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: PCR amplification and sequencing of Aspergillus niger phytase gene
[0027] 1. The present invention uses the phytase gene (PhyA) sequence of Aspergillus niger origin as a reference, designs and synthesizes two oligonucleotide primers downstream of the signal peptide, extracts Aspergillus niger total DNA by the method of phenol extraction, and passes PCR The target gene PhyA was amplified by the method, and the nucleotide sequence of the PCR product was determined.
[0028] The two PCR primers are as follows:
[0029] F1: TCTCTCGAGAAAAGTCCAAGTCCTGCGATACGGT
[0030] R1: AAGAATGCGGCCGCTTATCAACTAAAGCACTCTCCCC
[0031] The PCR reaction system is as follows:
[0032] Template
2ul
F1
1ul
R1
1ul
2×GC buffer I
5ul
high fidelity enzyme
0.5ul
DNTP
8uI
wxya 2 o
32.5uI
[0033] PCR program setting:
[0034] Pre-denaturation at 95°C for 5 minutes
[0035] 94°C, 30s; 65°C, 30s;...
Embodiment 2
[0038] Embodiment 2: the connection of phytase gene PHYA and cloning vector pPlCZαA
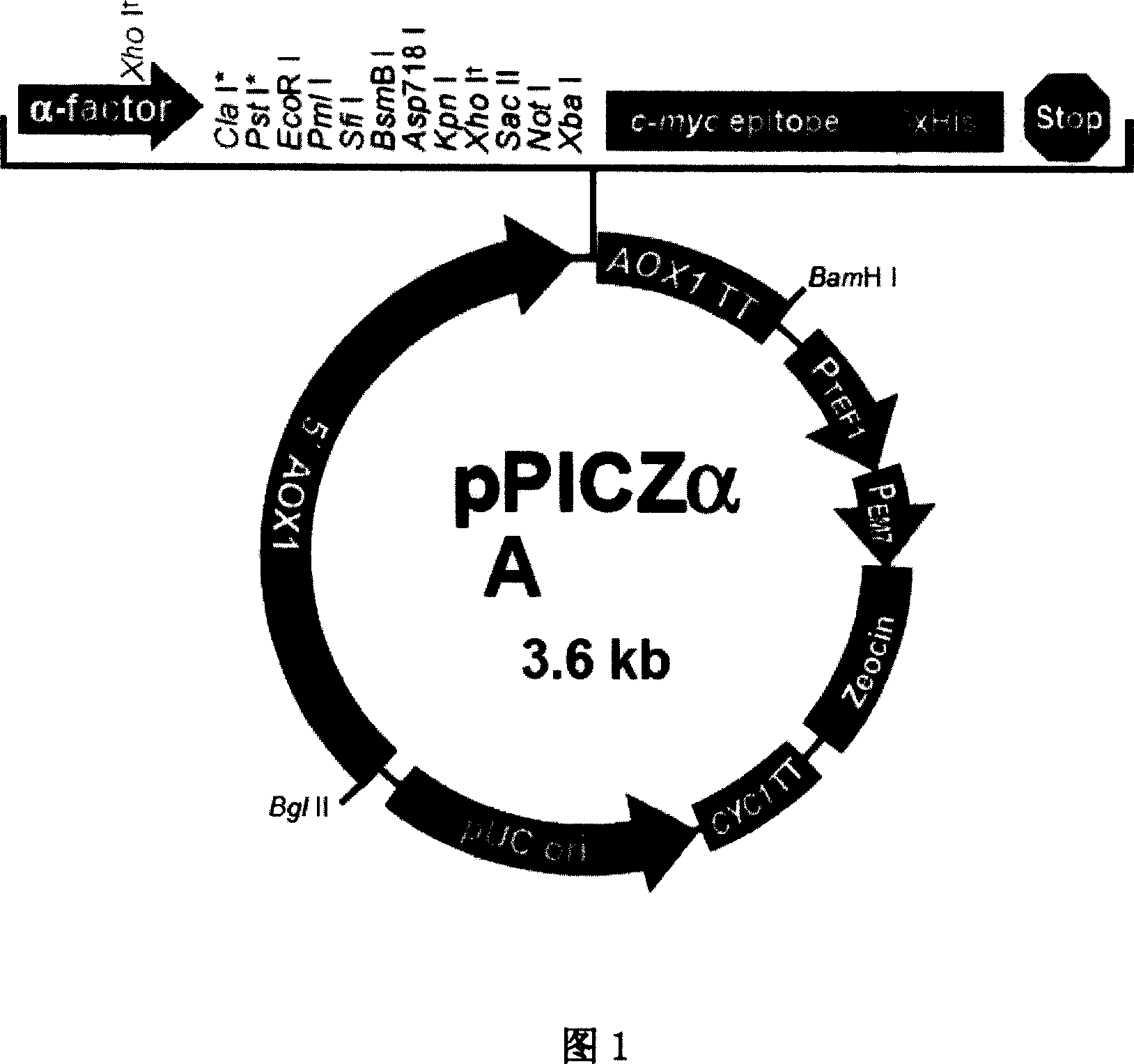
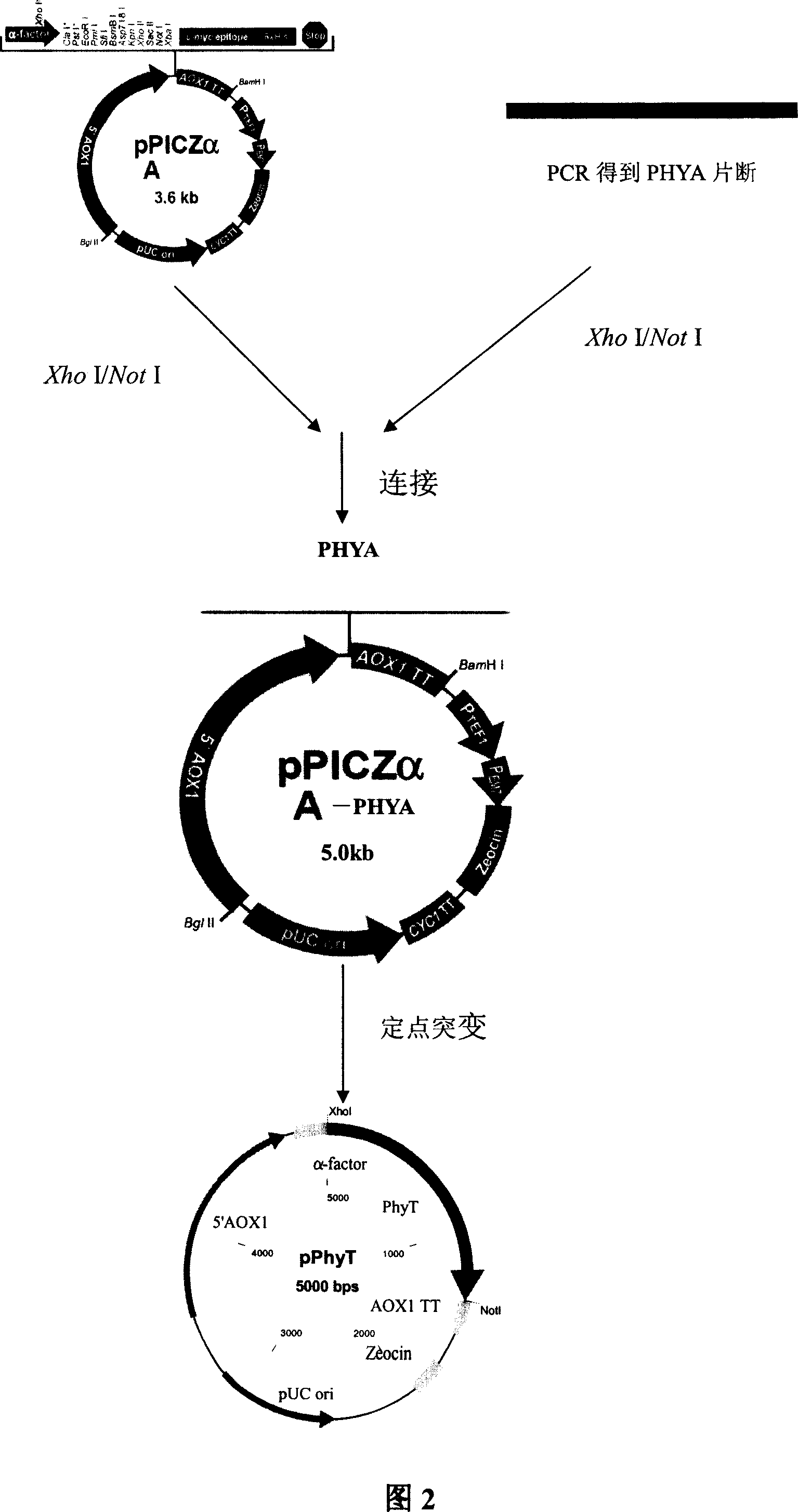
[0039] PhyA and the cloning vector pPlCZαA (as shown in Figure 1) were subjected to double digestion with restriction endonuclease Xho I / Not I respectively, and the digestion conditions were as follows:
[0040] PCR fragment digestion system (20ul)
[0041] Digest at 37°C for 10 hours, recover two target fragments after electrophoresis, and connect them with T4DNA ligase. The connection system is as follows:
[0042] PCR fragment
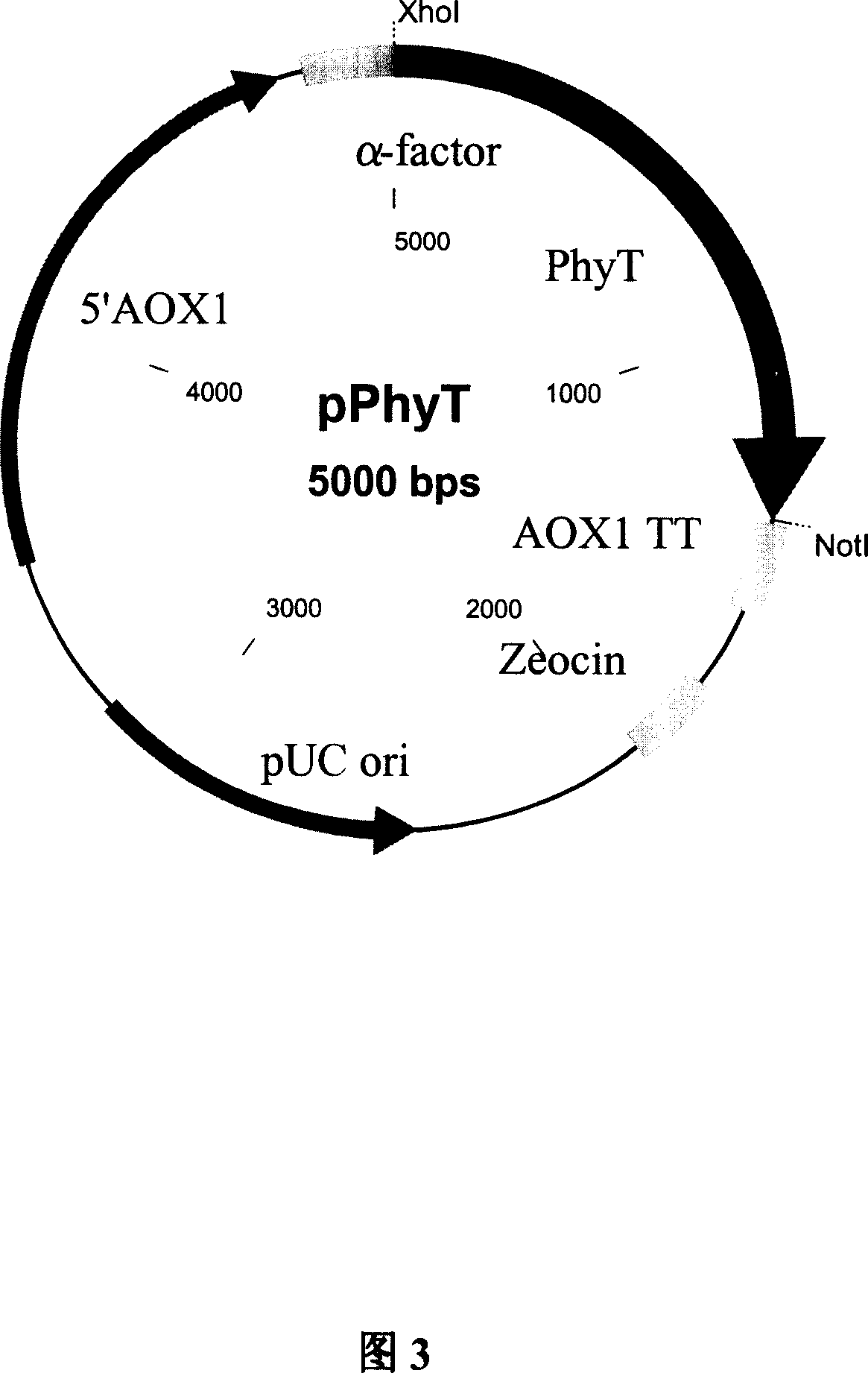
[0043] Ligated overnight at 16°C, the electrophoresis results showed a fragment of about 5kb, and the electrophoresis results after double digestion with Xho l / Not l showed two bands of 3.6kb and 1.4kb, indicating that the ligation was successful, and the pPhyA vector (PhyA is the code name of the phytase gene transformed by site-directed mutation), that is, pPICZαA-PHYA as shown in FIG. 3 .
Embodiment 3
[0044] Example 3: Site-directed mutagenesis
[0045] After repeated experiments and exploration, we determined to carry out site-directed mutagenesis on the amino acids at the following positions:
[0046] For the 23rd amino acid mutation, design the following primers:
[0047] F2: 5'TTGTACTCGCCATTCTTTTC3'
[0048] mutation site
[0049] R2: 5'GCCCCATAGATGAGAAGTCG3'
[0050] For the 53rd amino acid mutation, the following primers were designed:
[0051] F3: 5'TTGCGCCATGGAGCGCGGTA3'
[0052] mutation site
[0053] R3: 5'TAGCACCTGTACCAAGGTGA3'
[0054] For the 136th amino acid mutation, the following primers were designed:
[0055] F4: 5' TAC GGTCGGGTTATTGCTTC3'
[0056] mutation site
[0057] R4: 5'GCCTGAGGCGCGAATAAACG3'
[0058] For the 137th amino acid mutation, the following primers were designed:
[0059] F5: 5' GGT CGGGTTATGCTTCGGG3'
[0060] mutation site
[0061] R 5 : 5'GTAGCCTGAGGCGCGAATAA3'
[0062] For the 195th amino acid mutation, th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com