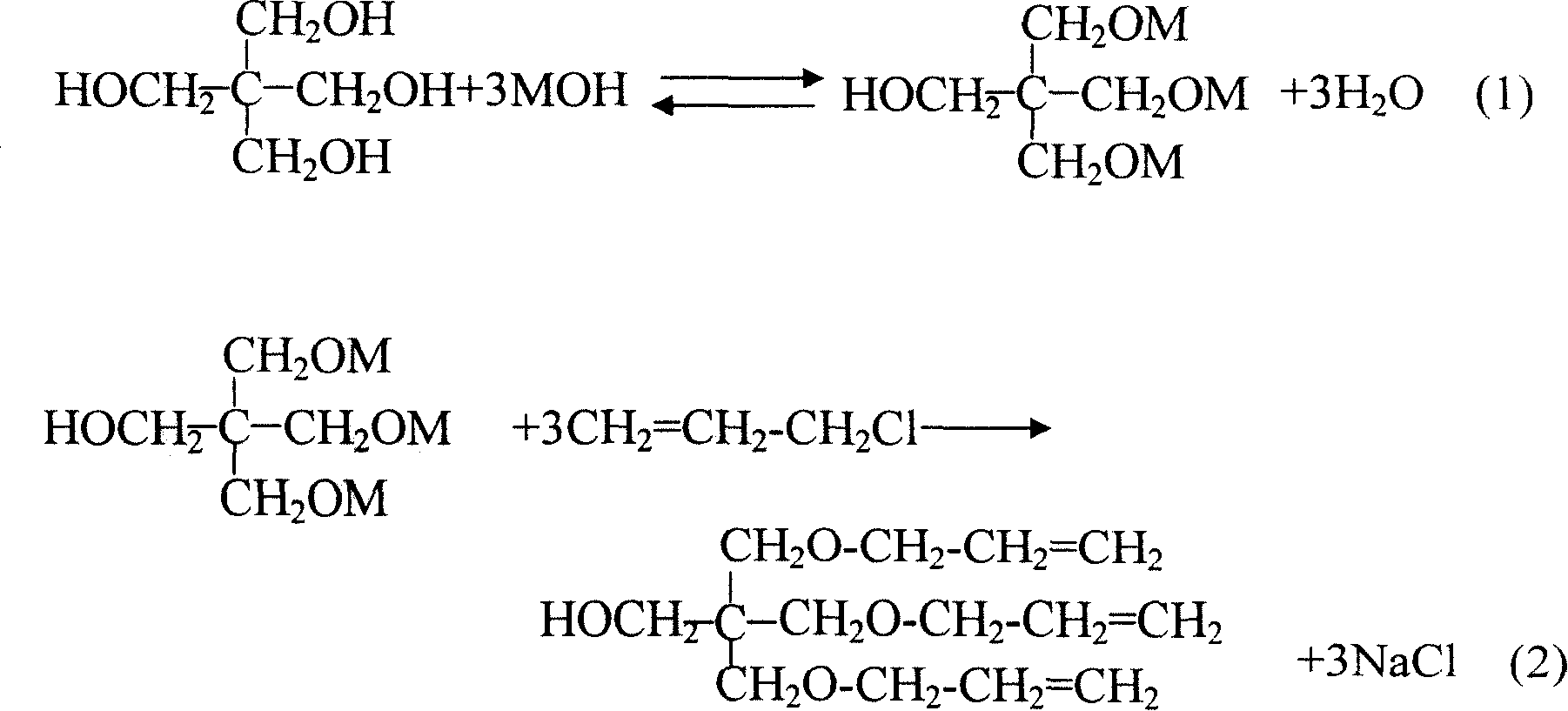Method for preparing pentaerythrite allyl ether
A technology of pentaerythritol allyl ether and pentaerythritol, which is applied in the field of preparation of pentaerythritol allyl ether, can solve the problems of difficult post-processing, large investment in equipment, insufficient contact, etc., and achieves easy industrial production, simple operation process, and suppression of side effects. The effect of the reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Raw materials and dosage:
[0030] Monopentaerythritol (>98%) 150g
[0031] Sodium hydroxide (99%) 150g
[0032] water 150g
[0033] Xylene 40g
[0034] Allyl chloride 281g (about 300ml)
[0035] Tetrabutylammonium bromide 4.5g
[0036] In a four-necked flask equipped with a reflux condenser, a stirrer, a thermometer and a dropping funnel, put monopentaerythritol, sodium hydroxide, water and xylene respectively, heat and stir to raise the temperature to 110°C. Start to add 1.5g of tetrabutylammonium bromide, and start to add 100ml of allyl chloride dropwise, control the drop rate slowly, and the dropwise addition is completed in about 1.5 hours; the reflux may be relatively large during the dropwise addition, and the temperature will drop to 90°C for a while, drop Halfway through, 1.0 g of tetrabutylammonium bromide was added. Pay attention to the continuous water separation when adding dropwise. After the dropwise addition of 100 ml of allyl chloride was complet...
Embodiment 2
[0038] Raw materials and dosage:
[0039] Monopentaerythritol (>98%) 150g
[0040] Sodium hydroxide (99%) 150g
[0041] water 150g
[0042] Xylene 40g
[0043] Allyl chloride 281g (about 300ml)
[0044] Benzyltriethylammonium chloride 4.5g
[0045]Process and equipment are with embodiment 1. Final product: 179g of product was obtained by distillation, wherein the content of diether was 4.09%, the content of triether was 81.4%, the content of tetraether was 14.51%, and the molar yield was 63.4% based on pentaerythritol.
Embodiment 3
[0047] Raw materials and dosage:
[0048] Monopentaerythritol (>98%) 300kg
[0049] Sodium hydroxide (99%) 300kg
[0050] Water 600kg
[0051] Tetrabutylammonium bromide 13kg
[0052] Xylene 80kg
[0053] Allyl chloride 600kg
[0054] It is carried out in a pilot test kettle equipped with a reflux condenser (condensed with an ice machine), a heating mantle, a thermometer, an agitator, a manhole, and a feeding port. Clean the reaction kettle to check whether the equipment is in good condition, turn on the ice machine, and pour 300kg of monopentaerythritol, 300kg of sodium hydroxide, 200kg of water, 80kg of xylene, and 5kg of tetrabutylammonium bromide according to the formula. After feeding the material, cover the manhole cover, raise the temperature, and stir in a jog. After the stirring is in normal operation, stir the material evenly. When the temperature of the kettle rose to about 110°C, the allyl chloride was slowly added dropwise. Depending on the temperature of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com