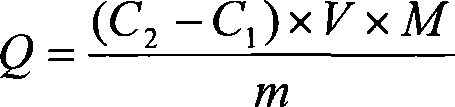Method for preparing etherification luffa and application of it in metallic ion adsorption
A loofah, etherification technology, applied in chemical instruments and methods, adsorption water/sewage treatment, other chemical processes, etc., can solve the problems of high development cost, environmental pollution, secondary pollution, etc., and achieve low price and environmental friendliness. , the effect of increasing revenue
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Alkali treatment: cut the natural loofah into small pieces, crush it with a pulverizer and sieve it, mix 20-30 mesh loofah with an appropriate amount of 30% NaOH ethanol solution, and alkalize it at 25°C 24h; then transferred to a three-necked flask, refluxed in an 80°C constant temperature water bath for 3h, washed 4 times with deionized water until neutral, and dried at 70°C after suction filtration to obtain the alkali-treated loofah;
[0034] Preparation of etherified loofah: 2.4g alkali-treated loofah is fully dispersed in a 250mL three-necked flask with an appropriate amount of 8% NaOH solution, and the alcohol solution of monochloroacetic acid is gradually added dropwise. Monochloroacetic acid and Alkali-treated loofah with a molar ratio of 5:1, reacted at 70°C for 5h, cooled to room temperature, filtered with suction, discarded the reaction solution, washed 5 times with deionized water until neutral, and dried at 70°C , to obtain etherified loofah. The physical...
Embodiment 2
[0036] Alkali treatment: cut the natural loofah into small pieces, sieve after pulverizing with a pulverizer, mix 20-30 mesh loofah with an appropriate amount of 30% NaOH ethanol solution in mass percentage, and transfer it to a three-necked flask. Intermittently irradiate with 600W microwave for 5 minutes; then transfer to a three-necked flask, reflux in 80°C constant temperature water bath for 3h, wash with deionized water 4 times until neutral, and dry at 70°C after suction filtration to obtain alkali-treated loofah ;
[0037] Preparation of etherified loofah: 2.4g alkali-treated loofah is fully dispersed in a 250mL three-necked flask with an appropriate amount of 8% NaOH solution, and the alcohol solution of monochloroacetic acid is gradually added dropwise. Monochloroacetic acid and Alkali-treated loofah with a molar ratio of 5:1, reacted at 70°C for 5h, cooled to room temperature, filtered with suction, discarded the reaction solution, washed 5 times with deionized water...
Embodiment 3
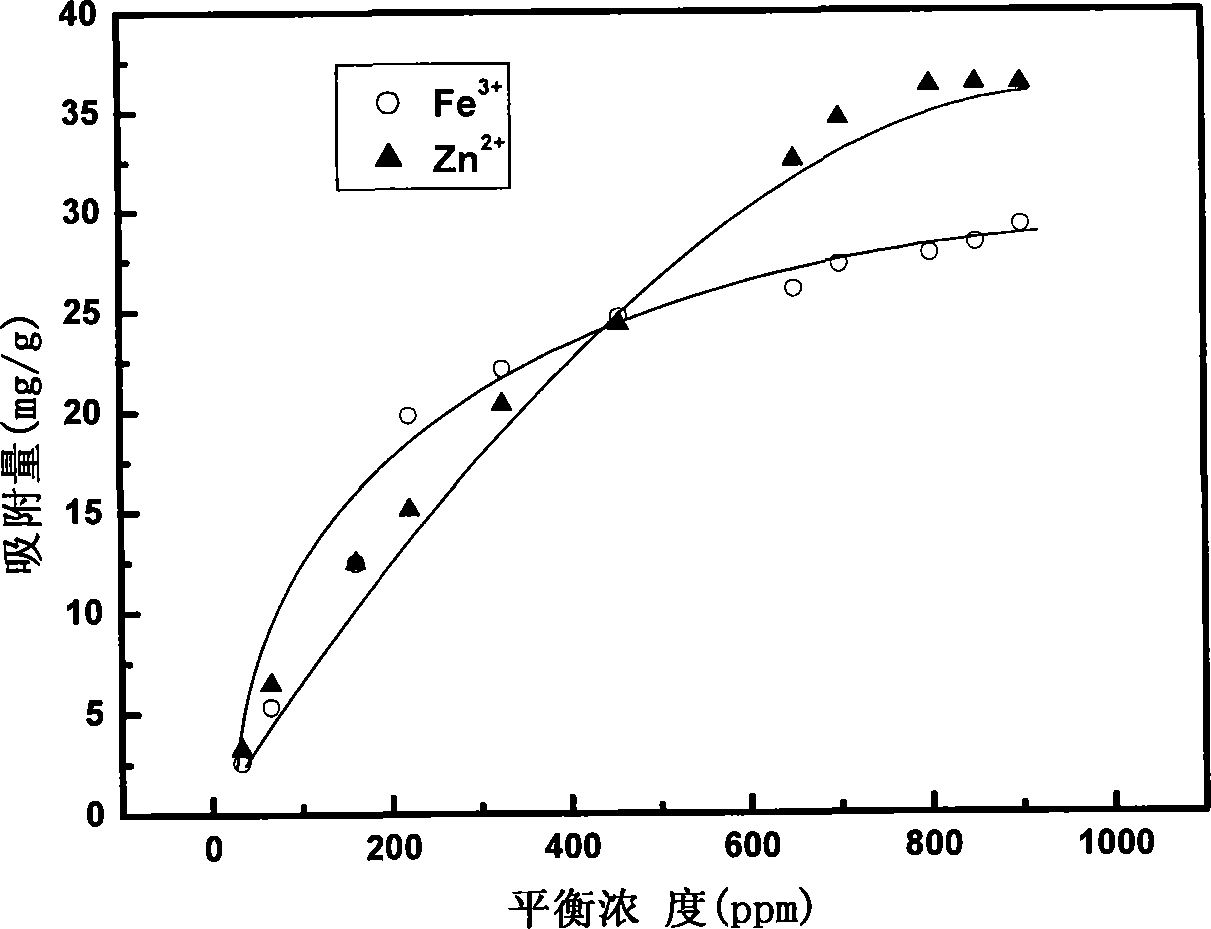
[0039] Determination of Adsorption Ability of Etherified Loofah on Mixed Metal Ions by Atomic Emission Spectrometry
[0040] (1) Take 1.5g of etherified loofah, transfer it to a three-necked bottle, add 150mL of metal ion mixed solution, add an appropriate amount of HCl or HAc dropwise, and place in an oil bath at 70°C for 6 hours. The etherified loofah was washed three times with deionized water, and dried at 80°C. for atomic emission spectrometry.
[0041] (2) In the adsorption experiment, three different mixed solutions of metal ions were used:
[0042] a) contains Ca 2+ 、Na + 、Cu 2+ , Mn 2+ 、Ba 2+ , Fe 3+ , Zn 2+ 、Cd 2+ Hydrochloride solution (the concentration of each metal ion is about 0.01M)
[0043] b) Contains ZnCl 2 , Pb(Ac) 2 , CdCl 2 The mixed solution (the concentration of each metal ion is about 0.005M)
[0044] c) Contains Pb(Ac) 2 , Co(NO 3 ) 2 , AgNO 3 The test results of the mixed solution (each metal ion concentration is about 0.01M) show t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com