Furoylureide penicillin crystal and preparation method thereof
A technology of furacillin and furacillin acid, which is applied in the field of semi-synthetic penicillin purification, crystallization and preparation, can solve problems such as difficult filtration and drying, poor product stability, and poor product quality, and achieve convenient transportation and storage , good industrial applicability, and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] According to the synthesis method of USP 3959258, 300 mL of 0.148 mol / L ethyl fururonyl penicillin acetate solution was prepared, cooled in an ice-salt bath to 0 ° C, and 0.5 mol / L sodium carbonate solution was added dropwise to make the pH = about 7, and stirred for 10 min , stand still for phase separation, remove ethyl acetate, move the water phase into a reaction flask, add 200 mL of ethyl acetate, dropwise add 3N hydrochloric acid to adjust pH=2.5, stir for 10 min, stand still for phase separation; extract once with 50 mL of ethyl acetate, combine The organic phase was dried by adding anhydrous magnesium sulfate. Filter, add 100 mL of petroleum ether dropwise with stirring, and grow the crystal for 30 minutes; then continue to add 200 mL of petroleum ether dropwise, and stir for 2 hours. Cool to 5°C in an ice-water bath, filter, wash and dry the slurry, and dry in vacuum to obtain the product with a yield of 89.2%.
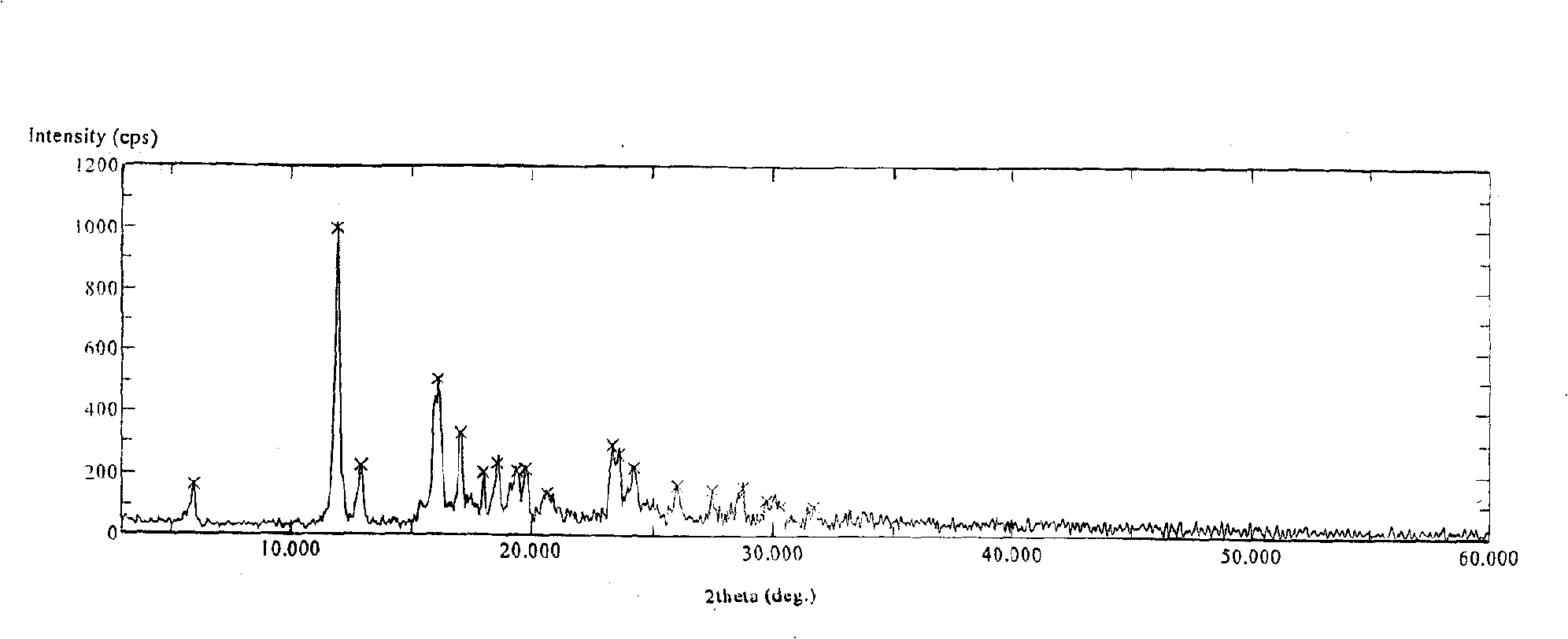
[0025] Use D / max-IIIA DIFFRATOMETER (RIGAKU COR...
Embodiment 2
[0030] The reaction product prepared according to the synthesis method of Example 1 was 300 mL of 0.137mol / L fururacillin acid dichloromethane solution, and the obtained fururacil acid dichloromethane solution was cooled to 0° C. in an ice-salt bath, and 0.5 mol / L sodium bicarbonate solution to make the pH=7, stir for 10min, stand still and separate the phases, transfer the water phase into the reaction flask, add 200mL of dichloromethane, add 2N sulfuric acid dropwise below 0°C to adjust the pH=2, stir for 10min, Stand still and separate the phases; the aqueous phase was extracted once with 50 mL of dichloromethane, the organic phases were combined, and dried by adding anhydrous magnesium sulfate. Filter, add 100mL isopropyl ether dropwise for 30min while stirring, then add 200mL isopropyl ether dropwise, and stir for 2 hours. Cool to 5°C in an ice-water bath, filter, wash and dry the slurry, and dry in vacuum to obtain the product with a yield of 86.3%. Measured X-ray powde...
Embodiment 3
[0032] According to the synthetic method of ("Chinese Journal of Medicinal Chemistry" 2006, 2, 16 (1), 51), 300 mL of 0.137 mol / L fururonyl penicillin acid trichloromethane solution was prepared, cooled to 0 ° C in an ice-salt bath, and slowly added dropwise 0.5 mol / L sodium hydroxide solution to make the pH = about 7, control the temperature, then add 200mL ice water, stir for 10min, let stand to separate the phases, move the water phase into the reaction bottle, add 200mL chloroform, and cool in an ice-salt bath to Below 0°C, slowly add 2N phosphoric acid dropwise to adjust the pH to 2, stir for 10 minutes, and let stand to separate the phases; extract the aqueous phase once with 50 mL of chloroform, combine the organic phases, and add anhydrous magnesium sulfate to dry. Filter, add 100mL isopropanol dropwise with stirring, grow the crystal for 30min, then continue to add 200mL isopropanol dropwise, and stir for 2 hours. Cool to 5°C in an ice-water bath, filter, wash and dry...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com