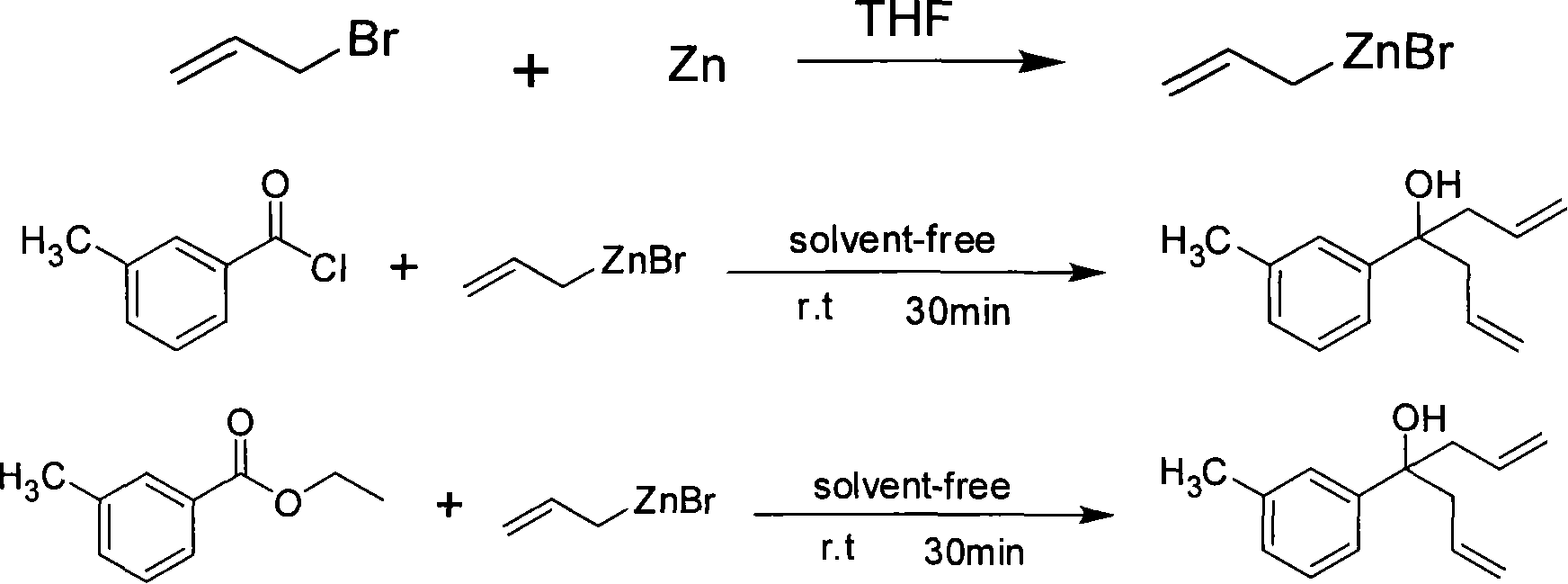
Method for preparing bis allyl alcohol compound 4-(3-methyl phenyl)-1,6-heptadiene-4-alcohol
A technology of methyl phenyl and allyl zinc bromide is applied in the preparation of hydroxyl compounds, the preparation of organic compounds, chemical instruments and methods, etc. Mild conditions, lower synthesis costs, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] (1) Activation of zinc powder and preparation of allyl zinc bromide
[0016] Add zinc powder (0.65g, 10mmoL) into a dry round bottom flask (50mL) equipped with a dropping funnel and a ventilation device, replace the air in the bottle with nitrogen, and then add 1,2-dibromo Ethane (1mmoL) and tetrahydrofuran (2mL); heat the mixture to 65°C, react for 2-3 minutes, cool to room temperature, add trimethylchlorosilane (1mmoL), stir for 15 minutes, then add allyl bromide (9mmol ) and tetrahydrofuran (2mL), the rate of addition was controlled, and the addition was completed in about 20 minutes, followed by stirring for 30 minutes to obtain a solution of allyl zinc bromide in tetrahydrofuran. Remove THF for use.
[0017] (2) Preparation of 4-(3-methylphenyl)-1,6-heptadien-4-ol
[0018] Slowly add m-toluoyl chloride (3nmol) dropwise from the dropping funnel at room temperature (the molar ratio of m-toluoyl chloride to allyl zinc bromide is 1:3), about 10 to 20 minutes after th...
Embodiment 2
[0032] (1) Activation of zinc powder and preparation of allyl zinc bromide
[0033] Same as Embodiment 1.
[0034] (2) Preparation of 4-(3-methylphenyl)-1,6-heptadien-4-ol
[0035]Slowly add m-toluoyl chloride (2.25nmol) dropwise from the dropping funnel at room temperature (the molar ratio of m-toluoyl chloride to allyl zinc bromide is 1:4), about 10 to 20 minutes after the drop, the reaction releases heat , continue stirring for 30 min, and the reaction is completed after the temperature drops to room temperature. After the reaction, add ether (10mL) and saturated ammonium chloride solution (15mL) into the reaction flask, stir for 10 minutes, and separate the organic phase; extract the aqueous phase with ether (10mL×3), combine the organic phases, and then Magnesium sulfate was dried, the solvent was evaporated and separated by column chromatography (silica gel, 300-400, petroleum ether / ethyl acetate=30 / 1) to obtain pure product 4-(3-methylphenyl)-1,6- Heptadien-4-ol. Th...
Embodiment 3
[0039] (1) Activation of zinc powder and preparation of allyl zinc bromide
[0040] Same as Embodiment 1.
[0041] (2) Preparation of 4-(3-methylphenyl)-1,6-heptadien-4-ol
[0042] Slowly add ethyl m-toluate (2.25nmol) dropwise from the dropping funnel at room temperature (the molar ratio of ethyl m-toluate to allyl zinc bromide is 1:4), drop it in about 10-20min, and react Heat was emitted, and stirring was continued for 30 min, and the reaction was completed after the temperature dropped to room temperature. After the reaction, add ether (10mL) and saturated ammonium chloride solution (15mL) into the reaction flask, stir for 10 minutes, and separate the organic phase; extract the aqueous phase with ether (10mL×3), combine the organic phases, and then Magnesium sulfate was dried, the solvent was evaporated and separated by column chromatography (silica gel, 300-400, petroleum ether / ethyl acetate=30 / 1) to obtain pure product 4-(3-methylphenyl)-1,6- Heptadien-4-ol. The assa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com