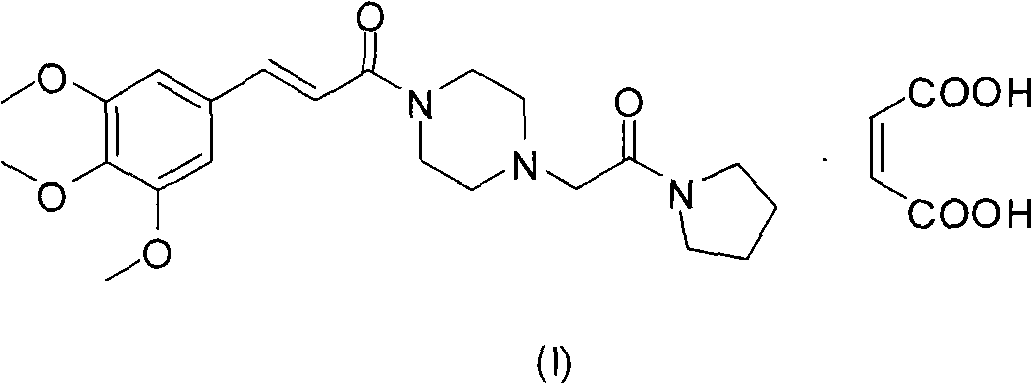
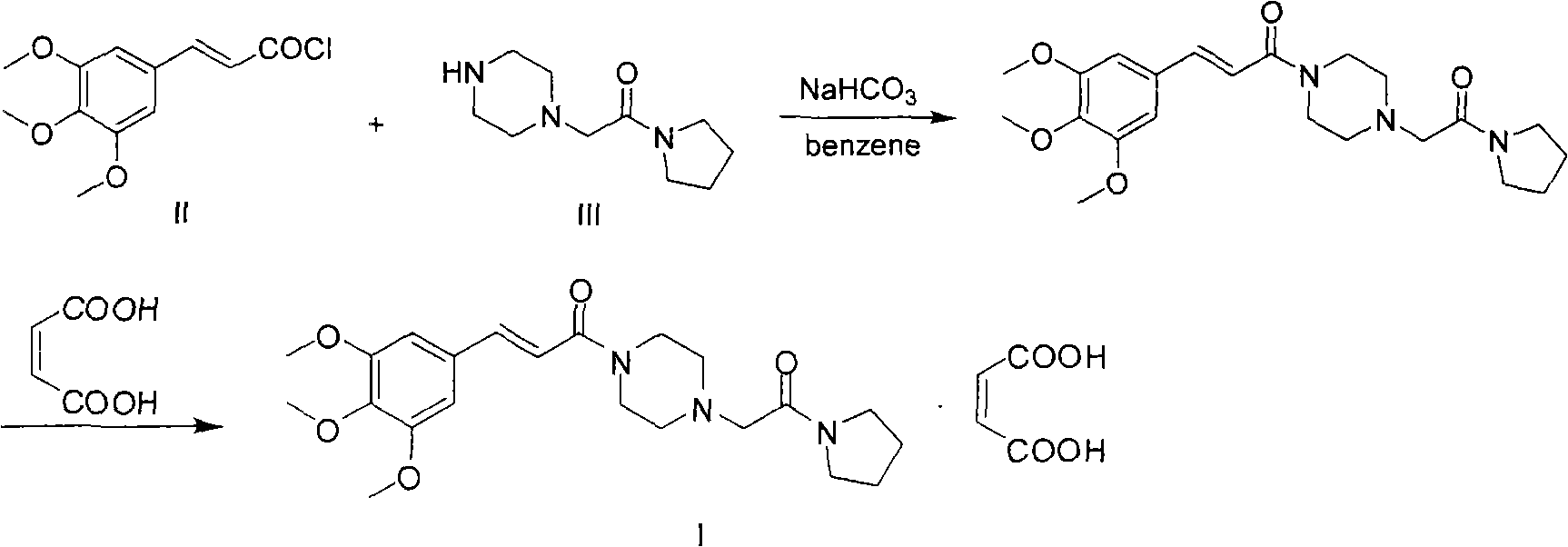
Method for preparing cinepazide maleate
A technology of cinepazide maleate and piperazine, which is applied in the field of preparation of cinepazide maleate, can solve the problems of many by-products of disubstituted piperazine, strong toxicity of solvent anhydrous benzene, and difficult purification of products, etc. , to achieve the effects of less pollution of three wastes, high product purity and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
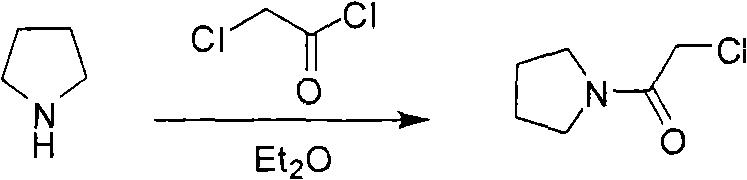
[0058] The synthesis of chloroacetylpyrrolidine (II), reaction formula:
[0059]
[0060] Chloroacetyl chloride (135g) and dichloromethane (340ml) were added to a 1-liter reaction flask, cooled to -10°C in an ice-salt bath, and triethylamine (127g), pyrrolidine (85g) and dichloromethane (340ml) were added dropwise ) mixed solution, control the temperature below 5°C, and complete the addition in about 2 hours. Stir at 0-5°C for 1 hour. Stir for an additional 1 hour at room temperature. Suction filtration, the filter cake was washed with dichloromethane (510ml), and dried to obtain a brown filtrate. After the filtrate was washed with water (150ml×3), it was concentrated to obtain 158g of a brown oily substance, with a yield of 90%.
Embodiment 2
[0062] Synthesis of step (1) 1-piperazine acetylpyrrolidine (III):
[0063] In a 2000ml reaction flask, add piperazine (206g) and water (340ml), stir to dissolve. Add hydrochloric acid (300ml) with a weight concentration of 30%, after stirring, add dropwise 400 g of an aqueous solution of chloroacetylpyrrolidine with a weight concentration of 40% at room temperature, stir and react for 6 hours, then add NaOH to adjust the pH of the system to 11. (450ml×3) extraction, combined extracts, concentrated under reduced pressure at 60°C 0.09MPa until no distillate came out to obtain a brown oily substance, steam distilled until no piperazine was found in the concentrate, stopped distillation, added 300ml toluene to heat After reflux and water separation until no water came out, the solid was filtered and dried to obtain 187.9 g of a light yellow powder with a melting point of 64° C. and a yield of 90%.
[0064] Step (2): Synthesis of 3,4,5-trimethoxycinnamoyl chloride (IV):
[0065]...
Embodiment 3
[0074] Using the same method as in Example 2, wherein, in step (1), the reaction time is 6 hours, the alkaline substance adopts KOH, and the pH is adjusted to 13, the product obtained in step (1) is 190g, and the melting point is 64°C. Yield 91%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com