Michlers ketone-cyano groups organic dyestuff and synthesis method thereof
A technology of organic dyes and synthesis methods, applied in the field of solar cells, can solve the problems of inability to popularize and expensive sensitizers, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
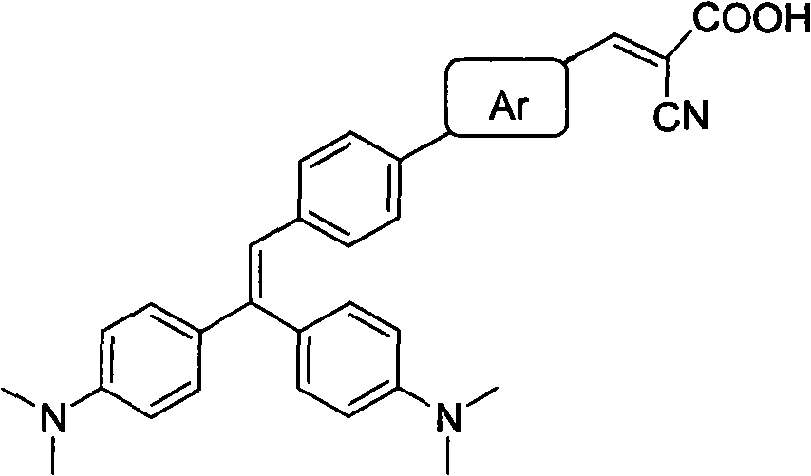
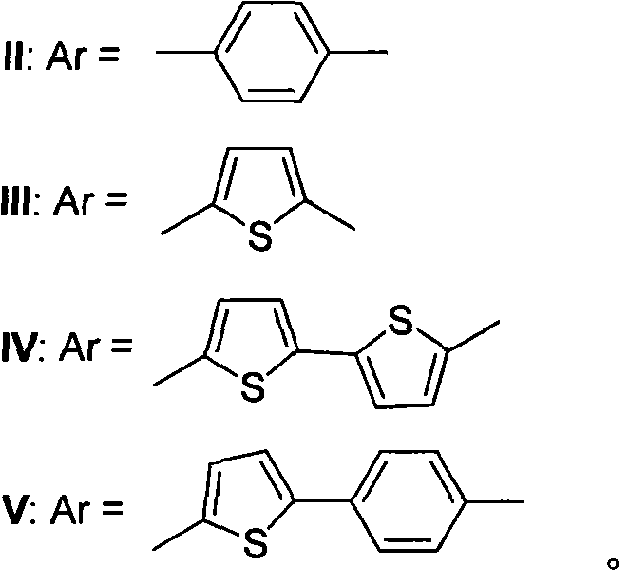
[0061] The synthetic route of novel Michler's ketone-cyano organic dye I:
[0062]
[0063] The synthesis method is:
[0064] Synthesis of Mie's Phosphate
[0065] Add 2.68g of Michler's ketone, 0.3g of sodium borohydride, 12mL of isopropanol and 0.7mL of water into a 50mL flask, heat to reflux for 4 hours, cool to room temperature, filter and wash to obtain Michler's alcohol, and recrystallize from ethanol to obtain pure rice Alcohol, productive rate 90%; Under the protection of nitrogen, add 2mL triethyl phosphite in 0.54g Michaelis alcohol (2.0mmol) at 0 ℃, then add 0.52g iodine (2.0mmol) in three batches, add Afterwards, the reaction mixture was continued to react at 0°C for 10 min, and at room temperature for 12 hours, adding 10% KOH aqueous solution and stirring for 30 min, separating the organic layer, extracting the aqueous layer with chloroform, combining the organic layers, washing with saturated brine, anhydrous Dried over magnesium sulfate, filtered and distil...
Embodiment 2
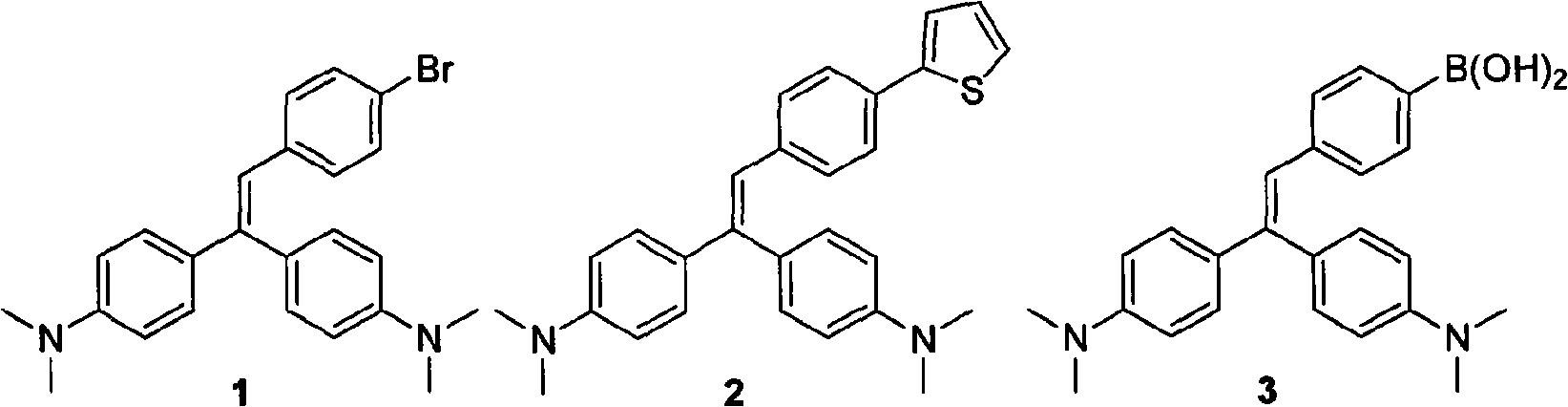
[0076] The synthetic route of novel Michler's ketone-cyano organic dye II:
[0077]
[0078] The synthesis method is:
[0079] Synthesis of Mie-substituted p-bromophenylalkenes 1
[0080] Under the protection of nitrogen, add 20 mL of THF and 0.66 g of potassium tert-butoxide to 1.5 g of Mie's phosphate (3.6 mmol) at 0°C, react at this temperature for 1 hour after the addition, and add 0.56 g of p-bromobenzene dropwise Dissolve formaldehyde in 10mL of THF solution, add saturated ammonium chloride aqueous solution after reacting at room temperature for 24 hours, separate the organic layer, extract with ethyl acetate, combine the organic layer, dry the organic layer with anhydrous magnesium sulfate, filter, The solvent was evaporated, and the solid was recrystallized from petroleum ether (60°C-90°C) to obtain Mie-substituted p-bromophenylalkene 1 with a yield of 60%.
[0081] Synthesis of Mie-substituted vinylphenylboronic acid 3
[0082] Under the protection of nitrogen, ...
Embodiment 3
[0090] The synthetic route of novel Michler's ketone-cyano organic dye III:
[0091]
[0092] The synthesis method is:
[0093] Synthesis of Mie-substituted p-bromophenylalkenes 1
[0094] Under the protection of nitrogen, add 20 mL of THF and 0.66 g of potassium tert-butoxide to 1.5 g of Mie's phosphate (3.6 mmol) at 0°C, react at this temperature for 1 hour after the addition, and add 0.56 g of p-bromobenzene dropwise Dissolve formaldehyde in 10mL of THF solution, add saturated ammonium chloride aqueous solution after reacting at room temperature for 24 hours, separate the organic layer, extract with ethyl acetate, combine the organic layer, dry the organic layer with anhydrous magnesium sulfate, filter, The solvent was evaporated, and the solid was recrystallized from petroleum ether (60°C-90°C) to obtain Mie-substituted p-bromophenylalkene 1 with a yield of 60%.
[0095] Synthesis of Mie-substituted p-thiophene phenylalkenes 2
[0096] Weigh 1.08g of activated magnesiu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com