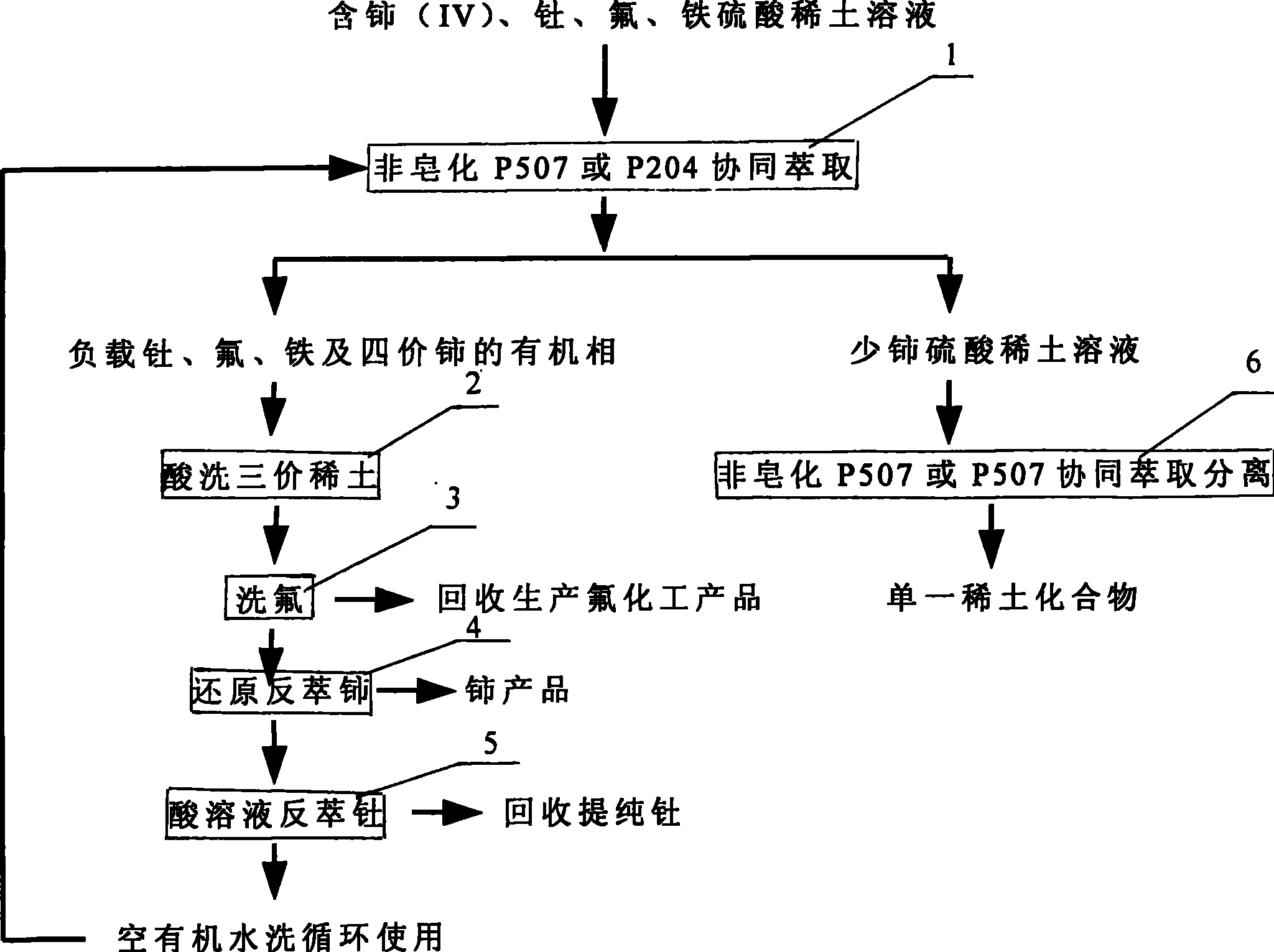
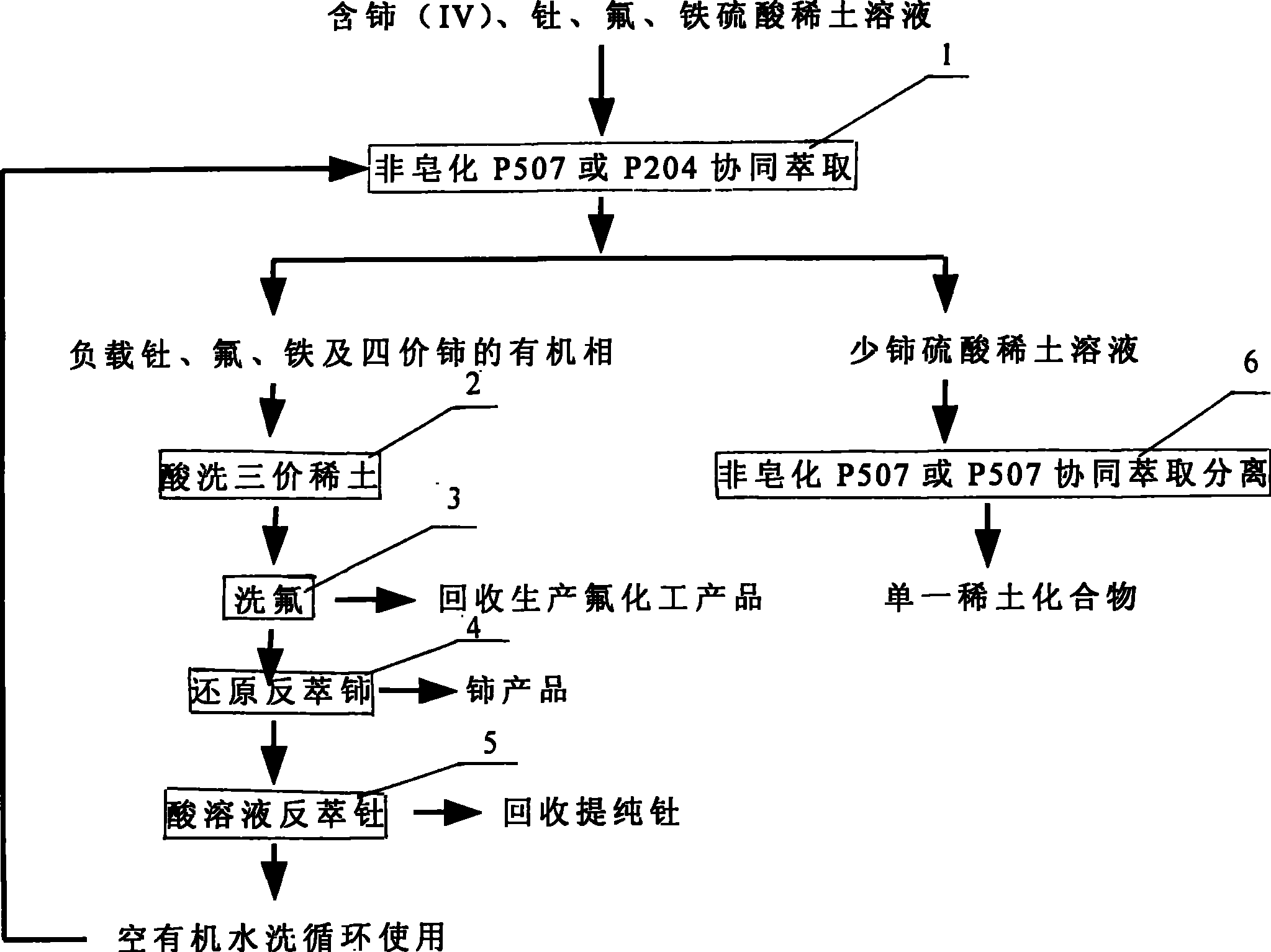
Technique for extraction separation of quadravalence cerium, thorium, fluorine and cerium less tervalence rare earth from sulphuric acid rare earth solution
The invention relates to a rare earth sulfate and a process method, which are applied in the fields of fluorine and cerium-less trivalent rare earth, thorium, extraction and separation of cerium, and non-saponification synergistic extraction agent for extraction and separation, which can solve the problem of large loss of extractant, low purity, and poisoning of the extractant. and other problems, to achieve the effect of increasing the yield of rare earth, simple process flow and large extraction capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The mixed ore of bastnaesite and monazite is roasted with sodium carbon and leached with sulfuric acid, and the rare earth sulfate solution containing high valence cerium, fluorine, thorium and iron is used as raw material, and Ce in the raw material 4+ / ∑Ce is 93% (weight percent), the rare earth concentration REO is 0.3 mol / liter, H + Concentration is 0.5 mol / L, F - at a concentration of 0.1 mol / L, ThO 2 The concentration of Fe is 0.1 g / L, and the concentration of Fe is 0.1 g / L. The extraction agent is a mixed extraction agent of 90% P507 and 10% TBP, the concentration is 1.0 mol / liter, the diluent is sulfonated kerosene, and the flow ratio used is organic phase: material liquid = 2: 1, after 3 stages of countercurrent extraction, Obtain the raffinate containing cerium (IV), fluorine, thorium and iron organic phase and trivalent rare earth;
[0035] The loaded organic phase after extraction is washed with 1 mol / L sulfuric acid through three stages of countercurrent...
Embodiment 2
[0041] Its process method and operation steps are the same as in Example 1. Rare earth sulfate solution containing high valence cerium, fluorine, thorium and iron after oxidative roasting-sulfuric acid leaching of bastnaesite is used as raw material, and the rare earth concentration REO in the raw material solution is 0.4 mol / liter , Ce 4+ / ∑Ce is 97%, H + At a concentration of 1.0 mol / L, ThO 2 The concentration is 0.50 g / L, F - The concentration of Fe is 0.8 mol / L, and the concentration of Fe is 3 g / L. The extractant is a mixed extractant of 95% P507 and 5% TRPO, the concentration is 1.5 mol / liter, the diluent is sulfonated kerosene, and the flow ratio is organic phase:material liquid=3:1. The loaded organic phase after extraction was washed with 5 stages of countercurrent with 3 mol / L sulfuric acid. Fluorine is washed with a nitric acid solution containing 2 mol / liter of aluminum salt and acidity of 1 mol / liter, so that 99% of the fluorine in the organic phase enters the...
Embodiment 3
[0043] Its processing method and operating steps are the same as in Example 1, and the rare earth concentration REO is 0.6 mol / liter in the raw material liquid, and the H + At a concentration of 1.0 mol / L, ThO 2 The concentration is 0.30 g / L, F - The concentration of Fe is 0.4 mol / L, and the concentration of Fe is 0.5 g / L. The extractant is a synergistic extractant prepared by 85% P204 and 15% TRPO, the concentration is 1.25 mol / L, and the loaded organic phase after extraction is washed with 2.5 mol / L sulfuric acid through three stages of countercurrent washing. Fluorine is washed with 1.5 mol / liter of aluminum salt nitric acid solution, the acidity is 0.5 mol / liter, and 99% of the fluorine in the organic phase enters the water phase. The loaded organic phase after fluorine washing is back-extracted cerium from the cerium-containing organic phase with 2.5% hydrogen peroxide and 4 mol / liter hydrochloric acid, and 6-stage countercurrent back-extraction, the tetravalent cerium ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| percent by volume | aaaaa | aaaaa |
| percent by volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com