Heparin affinity column and preparation method and use thereof
A technology of affinity and heparin, applied in the field of heparin affinity column and its preparation, can solve the problems of long reaction cycle, environmental pollution, and high toxicity, and achieve the effects of low cost, simplified preparation method, and reduced preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Required reagents: DEAE-cellulose-52, Sepharose 6FF, acrylamide, methylenebisacrylamide, SDS, sodium heparin, protein marker, Coomassie brilliant blue R250, sodium borohydride, rabbit anti-human apolipoprotein H Polyclonal Antibody, HClO 4 (72%-74%), (NH4) 2 SO 4 , TPB (0.039mol / L Tris-0.005mol / L H3PO4, pH8.6), 0.02mol / L Tris-HCl and other related reagents.
[0050] Preparation of affinity column:
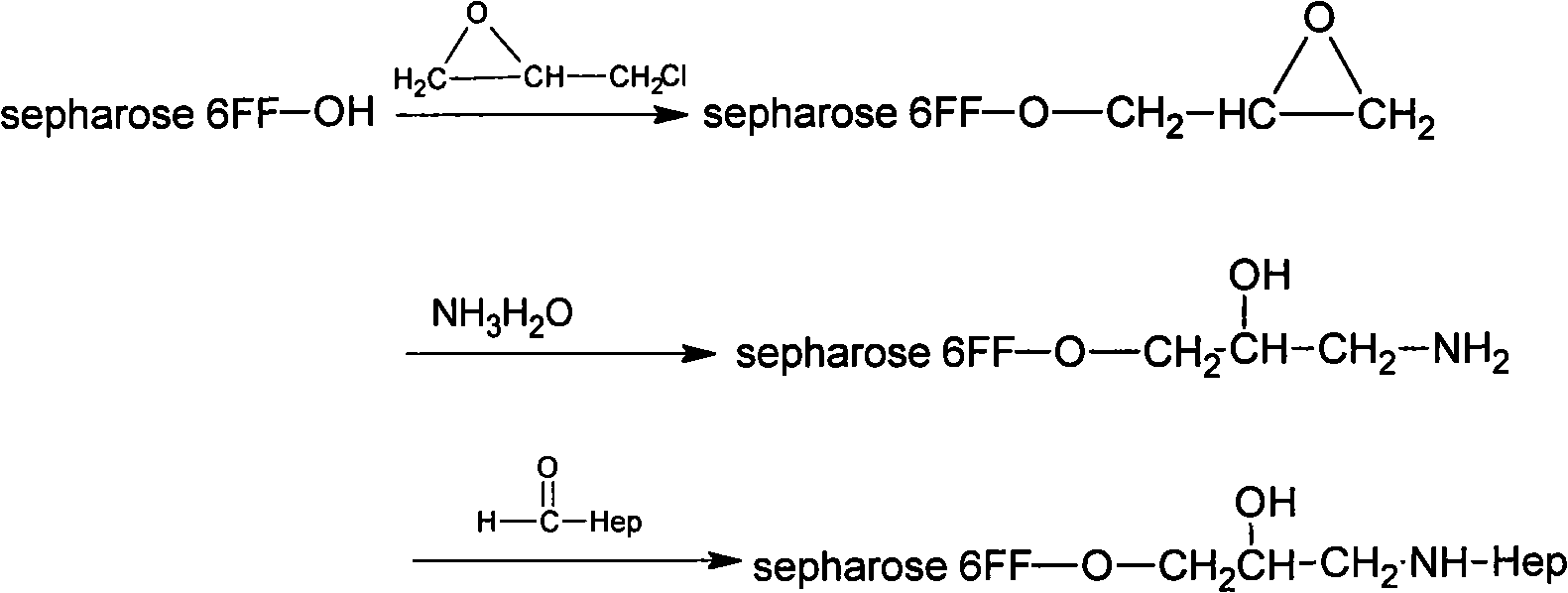
[0051] Take 20g of drained agarose gel 6FF, add 3ml of epichlorohydrin, 13ml of 2mol / L NaOH, and 30ml of distilled water, mix them, shake at 37°C for 2 hours, and obtain epoxidized agarose gel 6FF;
[0052] The obtained epoxidized agarose gel 6FF was washed with distilled water, dried and weighed to obtain about 18.5 g of gel.
[0053] Take 18g of epoxidized agarose gel 6FF and add 27ml of concentrated ammonia water to react at 37°C for 2 hours to obtain aminated agarose gel 6FF;
[0054] Wash the aminated agarose gel 6FF with distilled water, drain it and weigh it to o...
Embodiment 2
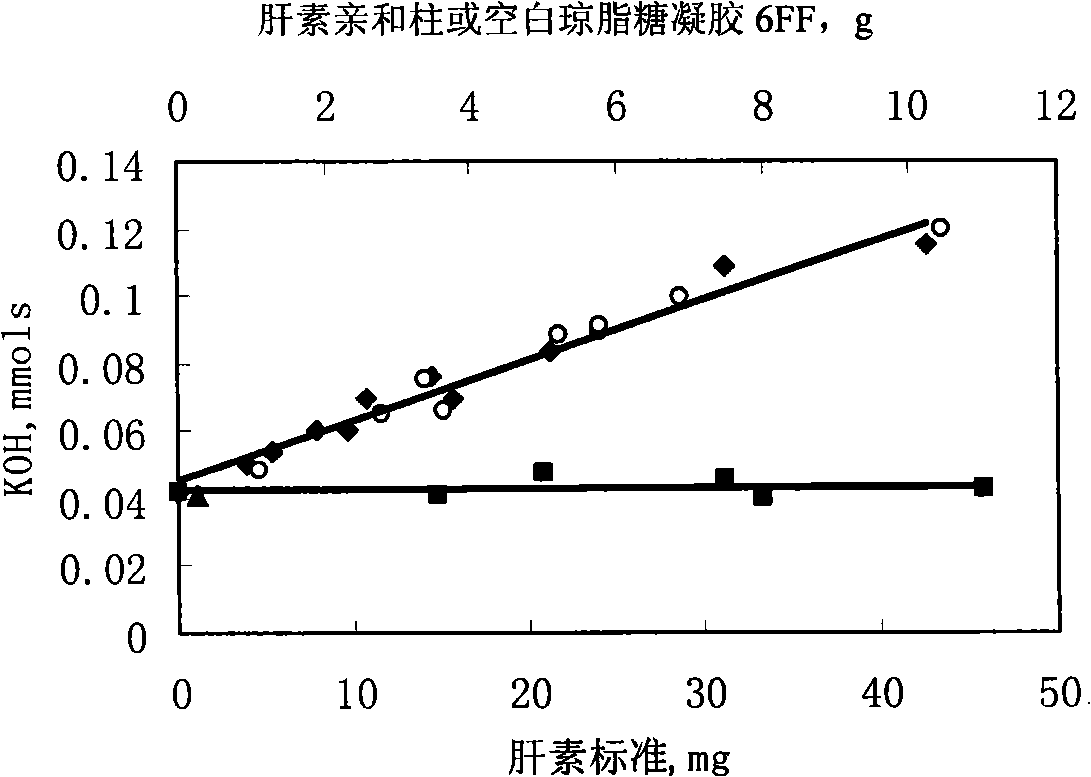
[0066] Preparation of the affinity column: the method was the same as in Example 1, using 30 g of Sepharose 6FF to prepare, and finally 26.3 g of Heparin Sepharose 6FF was stored at 4° C. in a 0.15 mol / L NaCl solution containing 0.01% thimerosal.
[0067] Heparin affinity column for the purification of apolipoprotein H:
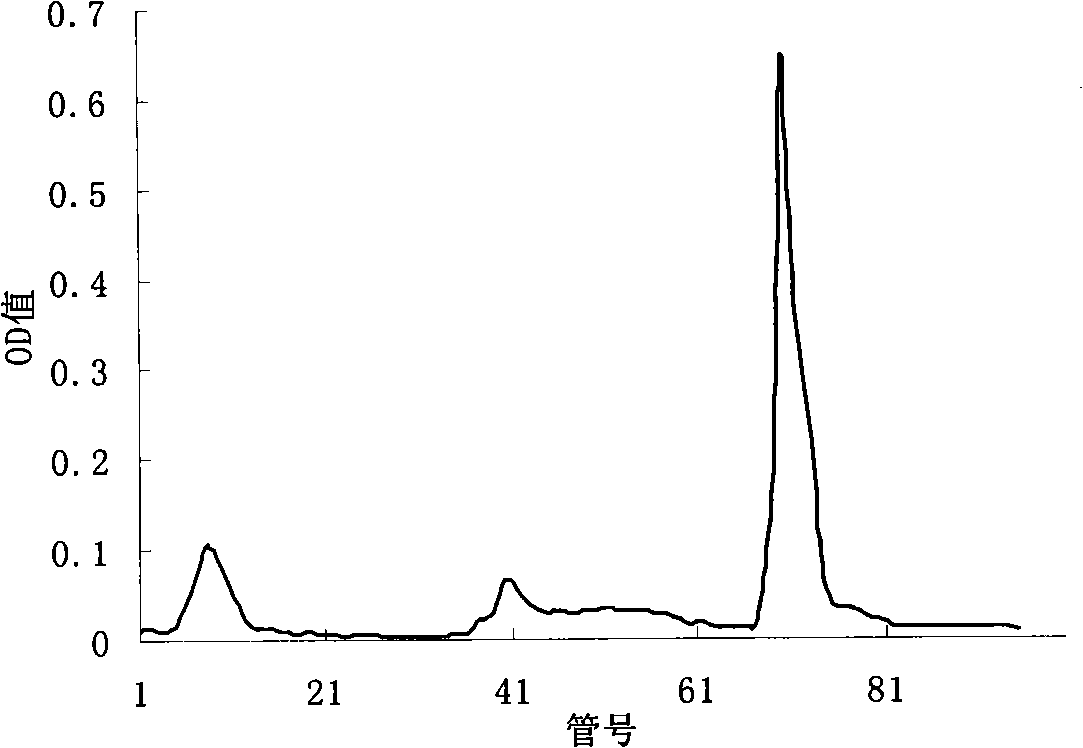
[0068] Preparation of Apolipoprotein H Crude Extract: Add 5.0mL 70% HClO to 200mL human mixed serum 4 (Volume / Volume), stirred at 4°C for 15 minutes, the properties changed from thin to thick and then to thin, centrifuged at 1000g for 15 minutes, and discarded the precipitate. After collecting the supernatant and adjusting the pH to neutral (pH 7.0-7.2) with 4mol / L NaOH, add 380g / L (NH 4 ) 2 SO 4 , overnight, and centrifuge to collect the precipitate. Precipitate with minimum volume TPB (0.039mol / L Tris-0.005mol / L H 3 PO 4 , pH 8.6) buffer solution, and after dialysis against TPB buffer solution, put on DEAE-cellulose-52 ion exchange column (1.7cm×32cm)...
Embodiment 3
[0073] The affinity column is used after activation and regeneration of the affinity column used in Example 2. The regeneration method is to rinse with 0.02mol / L Tris-HCl containing 1.5mol / L NaCl first, and then wash with 0.1mol / L NaOH;
[0074] Heparin affinity column for the purification of apolipoprotein H:
[0075] Preparation of apolipoprotein H crude extract: add 4.375mL 70% HClO to 175mL human mixed serum 4 (v / v), stirred at 4°C for 15 minutes, the property changed from thin to thick and then to thin, centrifuged at about 1500g for 15 minutes, and discarded the precipitate. After collecting the supernatant and adjusting the pH to neutral (pH7.0-7.2) with 4mol / L NaOH, add 380g / L (NH 4 ) 2 SO 4 , overnight, and centrifuge to collect the precipitate. Precipitate with minimum volume TPB (0.039mol / L Tris-0.005mol / L H 3 PO 4 , pH 8.6) buffer solution, and after dialysis against TPB buffer solution, put on DEAE-cellulose-52 ion exchange column (1.7cm×32cm) equilibrated w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com