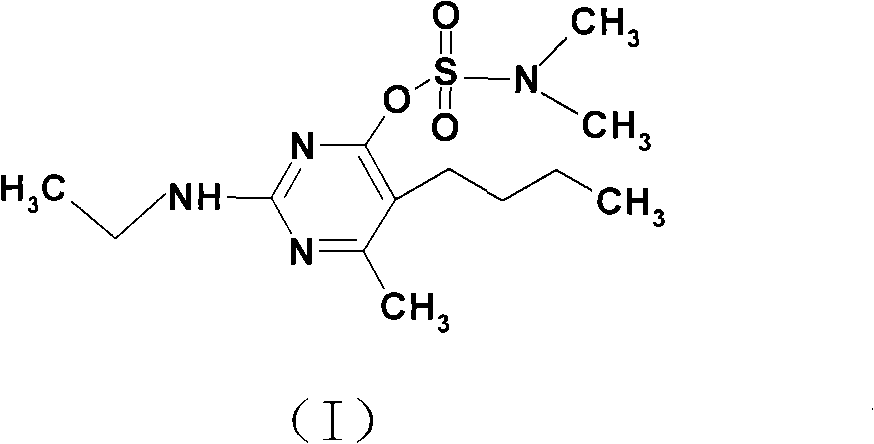
Method for synthesizing 5-nbutyl-2-ethylamido-6-methylpyrimidine-4-dimethyl amine sulfonic acid ester
A technology based on dimethyl sulfamate and methyl pyrimidine, applied in 5-n-butyl-2-ethylamino-6-methylpyrimidin-4-yl dimethyl sulfamate In the field of synthesis, it can solve the problems of low yield of the original drug, low purity of pyrimol, and low purity of the original drug, and achieve the effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
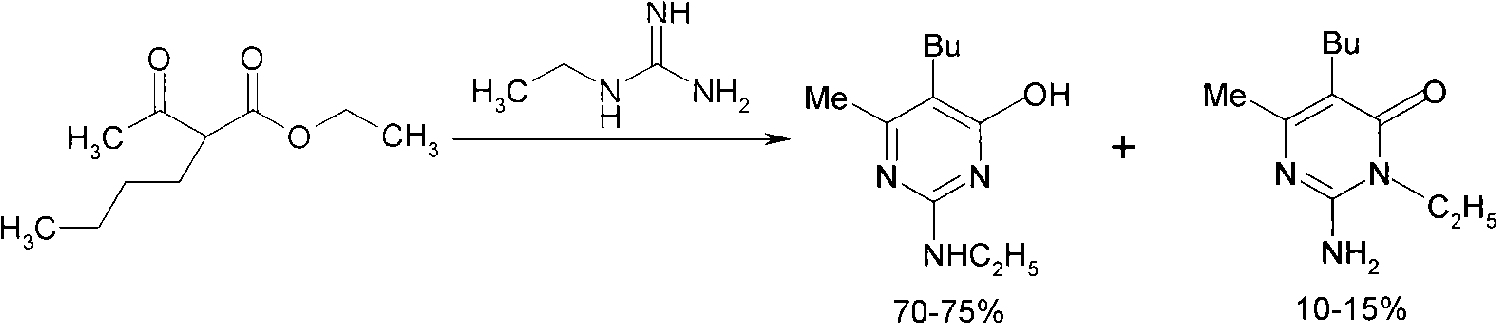
[0025] 1. Synthesis of 5-n-butyl-2-nitroamino-6-methylpyrimidin-4-ol
[0026] Add 180.0g (1.0mol) sodium methoxide / methanol solution (30%), 52.0g (0.5mol) nitroguanidine successively in a 1000ml three-necked flask, heat up to reflux, add 112.0g (0.6mol) 2-acetylhexanoic acid Ethyl ester, reflux for 8.0 hours, remove methanol by distillation under reduced pressure, add 300ml of water, stir for 0.5h, let stand for liquid separation, remove the organic layer, neutralize the aqueous layer with 10% hydrochloric acid to pH 5, white precipitate appears, filter to obtain 189.8g filter cake of 5-n-butyl-2-nitroamino-6-methylpyrimidin-4-ol, water content 45%, yield after drying 92.3%, melting point: 159℃~161℃, HPLC Chromatographic (HPLC) content 97.6%.
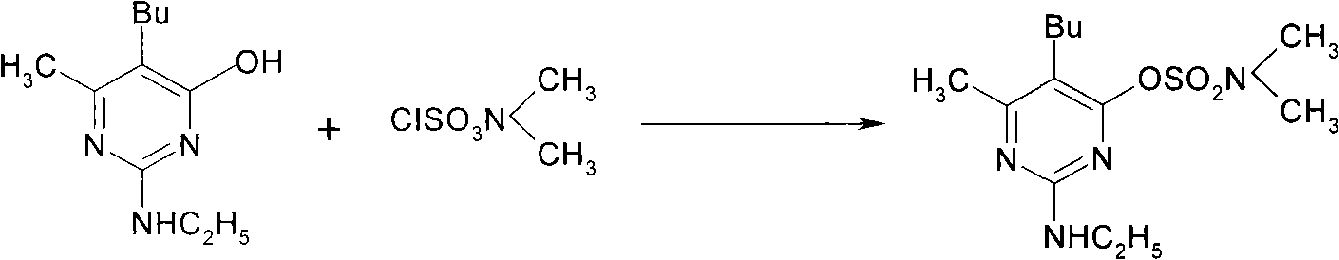
[0027] 2. Synthesis of pyrithrimol sulfonate
[0028] Add 48.4g (0.8mol) glacial acetic acid, 56.1g (0.8mol, 70%) ethylamine aqueous solution successively in 1000ml there-necked flask, stir at room temperature for 4.0h, add 500ml tolu...
Embodiment 2
[0036] 1. Synthesis of 5-n-butyl-2-nitroamino-6-methylpyrimidin-4-ol
[0037] The step is basically the same as step (1) in Example 1, except that sodium ethoxide / ethanol is used to replace sodium methoxide / methanol, 340.0g (1.0mol) sodium ethoxide / ethanol solution (20%) is added, and the reaction is refluxed for 4.0 hours , ethanol was distilled off under reduced pressure, 300ml of water was added, stirred for 0.5h, the organic layer was removed by static separation, and the aqueous layer was neutralized to pH 8 with 10% hydrochloric acid to obtain 186.4g of 5-n-butyl-2-nitroamine The base-6-methylpyrimidin-4-ol filter cake has a water content of 45%, a yield of 90.7% after drying, and an HPLC content of 95.3%.
[0038] 2. Synthesis of pyrithrimol sulfonate
[0039] The step is basically the same as step (2) in Example 1, and the difference is to replace toluene with xylene, sodium hydroxide to replace sodium carbonate, 5-n-butyl-2-nitroamino-6-methylpyrimidine- The molar r...
Embodiment 3
[0041] 1. Synthesis of 5-n-butyl-2-nitroamino-6-methylpyrimidin-4-ol
[0042] The steps are basically the same as step (1) in Example 1, except that the aqueous layer is neutralized to pH 3 with 10% hydrochloric acid to obtain 195.5g of 5-n-butyl-2-nitroamino-6-methyl The base pyrimidin-4-ol filter cake has a water content of 48%, a yield of 90.0% after drying, and an HPLC content of 96.2%.
[0043] 2. Synthesis of pyrithrimol sulfonate
[0044] Step is basically the same as step (2) in Example 1, and difference is to replace sodium carbonate with potassium carbonate, 5-n-butyl-2-nitroamino-6-methylpyrimidine-4-phenol, glacial acetic acid , ethylamine, acid-binding agent, and N, the molar ratio of N-dimethylsulfamoyl chloride is 1: 3: 3: 0.6: 1.2, and the target product obtained after purification is 106.6g, with a melting point of 47.4°C to 49.7°C, The yield is 84.2%, the GC content is 95.2%, and the total yield is 75.8% based on nitroguanidine as the starting material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com