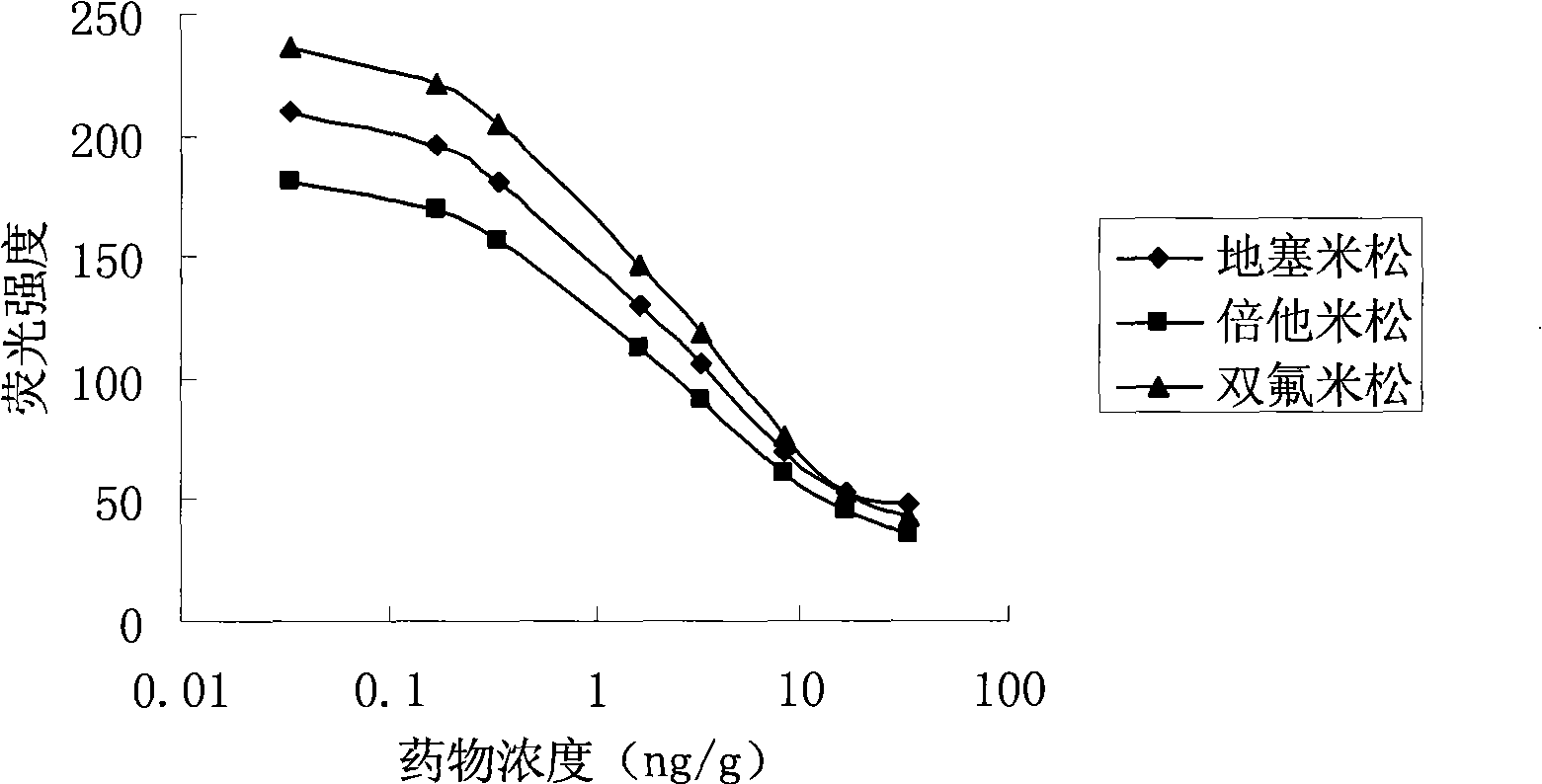
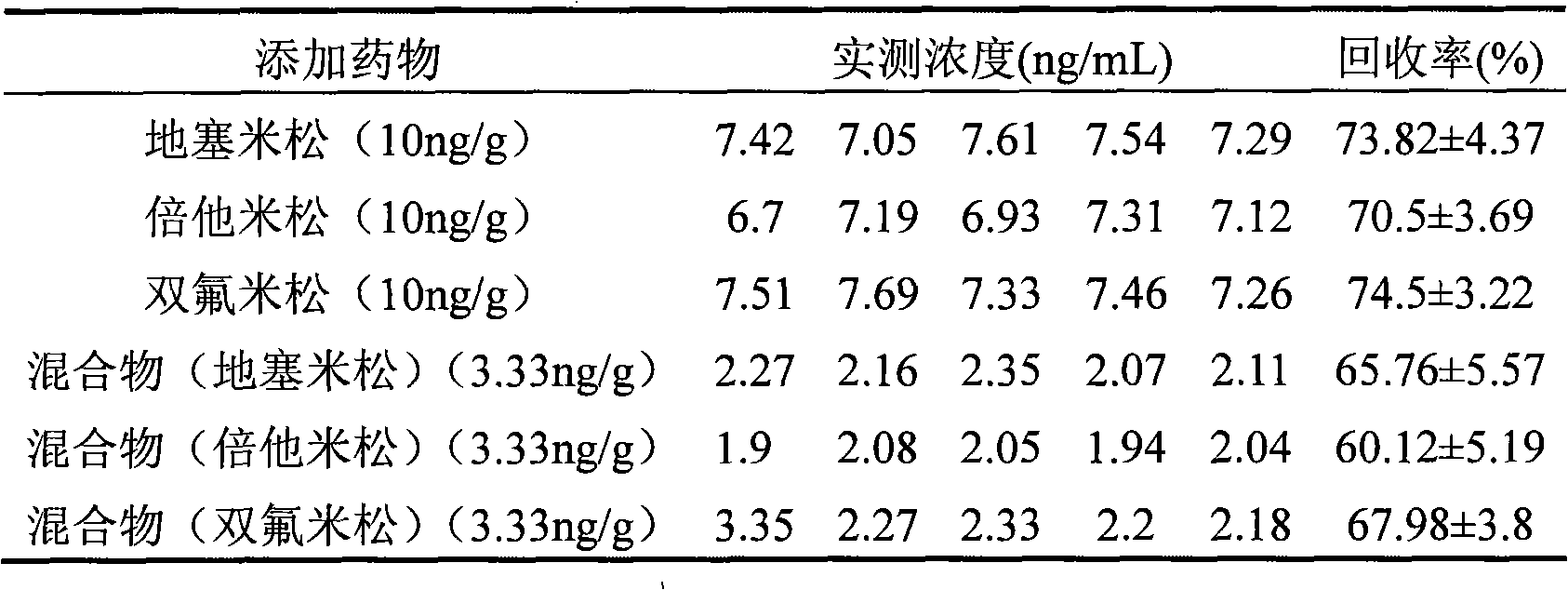
Method for quantum dot mark indirect competition fluoroimmunoassay detection for glucocorticosteroid residual
A technology of glucocorticoid and fluorescence immunity, which is applied in the field of immunoassay, can solve the problems of cumbersome operation and time-consuming, and achieve the effect of simple operation, strong fluorescence intensity and long fluorescence stability time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Coating three kinds of quantum dots QD545, QD590, QD650 with denatured bovine serum albumin (dBSA) labeled anti-dexamethasone, anti-betamethasone, anti-diflumetasone polyclonal antibodies
[0029] (1) BSA denaturation:
[0030] Dissolve 16.5mg BSA in 10mL double distilled water, add 0.42mg NaBH under stirring 4 , react at room temperature for 1h, heat at 60-80°C for 20min to decompose excess NaBH 4 , BSA is denatured, the disulfide bond is opened into -SH, and the concentration of the final dBSA aqueous solution is 5×10 -5 M.
[0031] (2) Denatured BSA-wrapped quantum dots (dBSA-QDs):
[0032] Mix dBSA and quantum dot chromium dysprosium (CdTe) in a molar ratio (1:1), heat in a water bath at 60-80°C for 15 minutes, and keep at room temperature for two days to completely wrap.
[0033] (3) Denatured BSA-coated quantum dot-coupled antibody
[0034] Mix 5 μL of 1-ethyl-(3-dimethylaminopropyl) carbodiimide EDC (0.056M) with 5 μL of sulfo-N-hydroxysuccinimide sulfo-...
Embodiment 2
[0041] Embodiment 2 adds recovery experiment:
[0042] (1) Extraction and purification of samples: add 10 mL of acetonitrile / water (7:3) mixed solution to 2 g of chicken meat samples, vortex for 1 min, ultrasonically sonicate for 30 min, centrifuge at 2000×g for 10 min, absorb 2.5 mL of the supernatant in a clean Add 4mL of n-hexane and 1mL of dichloromethane to degrease in a glass tube, vortex and mix for 1min to separate the three phases, draw 1mL of the intermediate phase (corresponding to 0.2g sample) into a clean test tube; at 50°C, slowly N 2 Blow dry, and dissolve the residue with 0.2mL standard diluent (phosphate buffered saline PBST containing Tween-20 containing 10% methanol), and use it as a sample for analysis.
[0043] (2) Add standard substance (dexamethasone, three kinds of mixtures of betamethasone and diflumetasone) liquid in the chicken sample of 2g blank, make its concentration be 10ng / g, each concentration prepares five parts of samples respectively, accord...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com