Preparation of docetaxel long-circulating liposome and freeze-dried powder injection thereof
A long-circulating liposome and docetaxel technology, which is applied to freeze-dried powder injection and its preparation, contains insoluble anticancer drug docetaxel long-circulating liposome field, can solve the problem of hindering liposome, stable It can improve the drug loading and stability, reduce unsafe factors, and have excellent storage stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
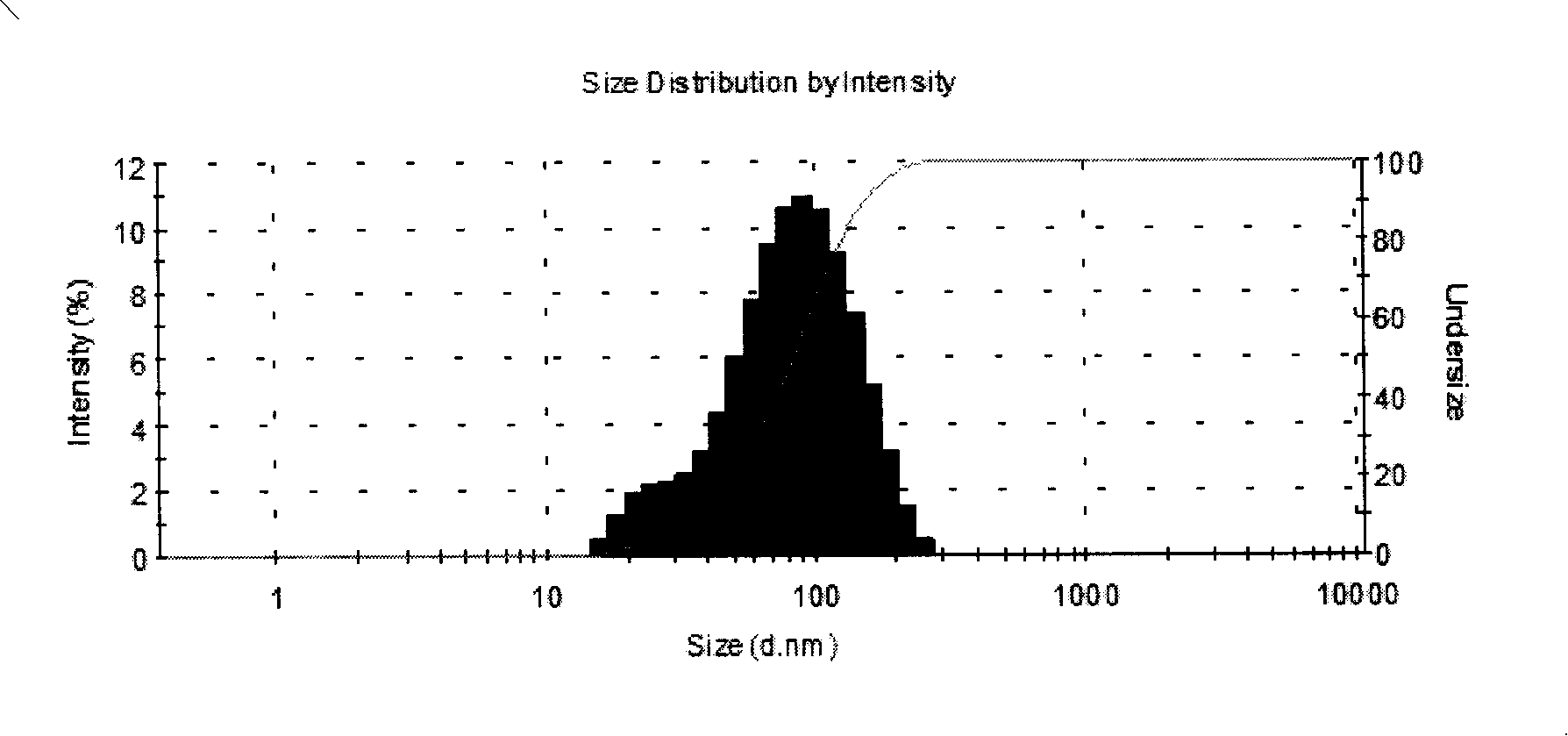
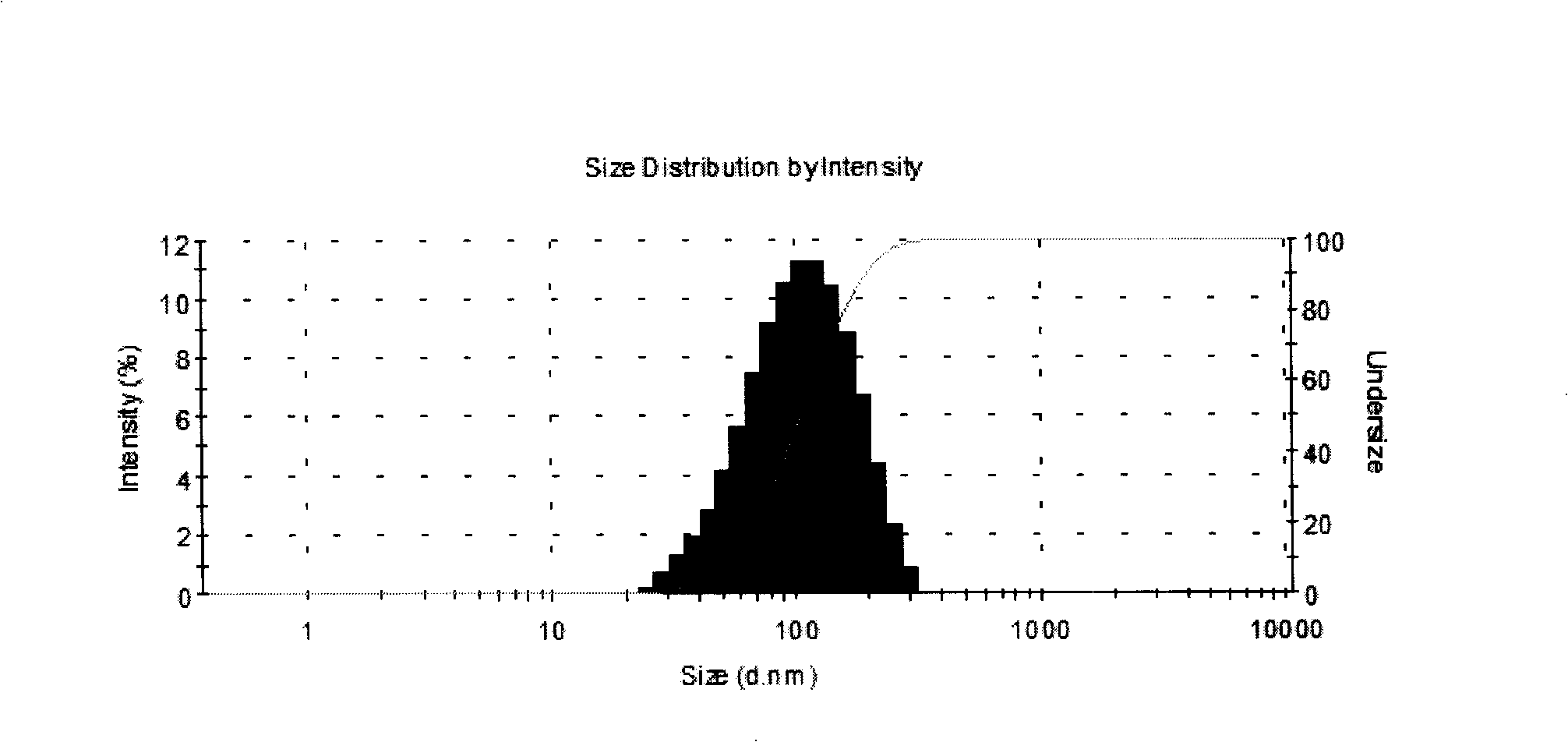
Image
Examples
Embodiment 1
[0054] Preparation prescription (100mL capacity)
[0055] Docetaxel 100mg
[0056] Soy Phospholipids (SPC) 1.5g
[0057] Distearoylphosphatidylglycerol (DSPG) 150mg
[0058] Cholesterol 600mg
[0059] a-Tocopherol 17mg
[0060] Sucrose about 10g
[0061] Sodium citrate about 294mg
[0062] Citric acid amount
[0063] Water for injection to the required volume
[0064] The preparation process is as follows:
[0065] Select soybean lecithin, distearoylphosphatidylglycerol, cholesterol and a-tocopherol in chloroform solution and mix uniformly according to the formula; use spray drying method to remove chloroform under reduced pressure to form lipid mixture; prepare 0.01M sodium citrate solution , Dissolving sucrose in sodium citrate solution as a hydration solution, the hydration temperature is generally between 55°C±5°C, multi-lamellar liposome suspension; after hydration is complete, use a high-pressure homogenizer to homogenize to an average The particle size is 100±1...
Embodiment 2
[0067] Preparation prescription (100mL capacity)
[0068] Docetaxel 200mg
[0069] Egg Yolk Lecithin (EPC) 4g
[0070] Dimyristoylphosphatidylglycerol (DMPG) 1g
[0071] Cholesterol 1g
[0072] a-tocopheryl succinate 90mg
[0073] Lactose about 10g
[0074] Sodium succinate about 800mg
[0075] Succinic acid amount
[0076] Water for injection to the required volume
[0077] The preparation process is as follows:
[0078] Select egg yolk lecithin, dimyristoyl phosphatidylglycerol, cholesterol and a-tocopheryl succinate to dissolve in chloroform-methanol (2:1) solution and mix well according to the formula; remove the organic solvent under reduced pressure by evaporation under reduced pressure , to form a lipid mixture; prepare 0.03M sodium succinate solution, dissolve lactose in sodium succinate solution as a hydration solution, the hydration temperature is generally 65 ° C ± 5 ° C, multi-lamellar liposome suspension; After the hydration is complete, use a high-pressu...
Embodiment 3
[0080] Preparation prescription (100mL capacity)
[0081] Docetaxel 400mg
[0082] Distearoylphosphatidylcholine (DSPC) 12g
[0083] Distearoylphosphatidylglycerol (DMPG) 2.4g
[0084] Cholesterol 6g
[0085] a-Tocopherol 100mg
[0086] Trehalose about 20mg
[0087] Phosphate about 1.5g
[0088] Water for injection to the required volume
[0089] The preparation process is as follows:
[0090] Select distearoylphosphatidylcholine, distearoylphosphatidylglycerol, cholesterol and a-tocopherol according to the formula and dissolve them in chloroform-methanol (2:1) and mix them uniformly; quality mixture; prepare 0.05M phosphate buffer solution, dissolve trehalose in phosphate solution as a hydration solution, the hydration temperature is generally 45°C±5°C, multi-lamellar liposome suspension; after complete hydration Use a high-pressure homogenizer to homogenize to an average particle size of 80 ± 10nm, dilute to the volume with water for injection and adjust to a concent...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com